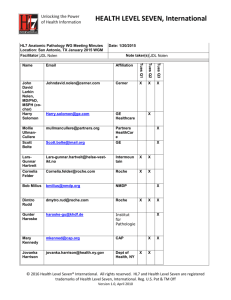
IT-014 Health Informatics Committee Final Report
advertisement

Final Report HL7 Meeting—Atlanta, USA (May 2013) IT-014 Health Informatics Committee Final Report HL7 Working Group Meeting 5–10 May 2013 (Atlanta, USA) Date Submitted: 30 July 2013 Lead Author: Dr Patricia Williams Collated by: Standards Australia With input from Australian Delegation and other employer funded Australians at the meeting: Richard Dixon Hughes (Delegate) Amy Mayer (Mentored Delegate) Dr Zoran Milosevic (Delegate) Dr Trish Williams (Delegate and Report Coordinator) Nat Wong (Delegate) Dr Stephen Chu (NEHTA) 1 Final Report HL7 Meeting—Atlanta, USA (May 2013) Contents 1. INTRODUCTION 6 1.1 OBJECTIVES OF MEETING 6 1.2 RELEVANCE TO NEHTA PROGRAMS 8 1.3 MEETING LOGISTICS 8 1.4 RECOMMENDATIONS ARISING FROM THE MEETING 12 1.5 FUNDING SOURCES SUMMARY AND AUSTRALIAN ATTENDANCE 20 1.6 AUSTRALIAN LEADERSHIP POSITIONS 20 2. WORKING GROUP REPORTS 22 2.1 ADVISORY COUNCIL 2.1.1 PROGRESS AT THIS MEETING 2.1.2 ACTIONS FOR AUSTRALIA 22 22 22 2.2 AFFILIATE AGREEMENT TASKFORCE 2.2.1 PROGRESS AT THIS MEETING 2.2.2 ACTIONS FOR AUSTRALIA 23 23 24 2.3 AFFILIATE DUE DILIGENCE COMMITTEE 2.3.1 PROGRESS AT THIS MEETING 2.3.2 ACTIONS FOR AUSTRALIA 25 25 25 2.4 ARCHITECTURE REVIEW BOARD 2.4.1 PROGRESS AT THIS MEETING 2.4.2 CURRENT PROJECTS 2.4.3 ACTIONS FOR AUSTRALIA 26 26 26 28 2.5 HL7 BOARD 2.5.1 PROGRESS AT THIS MEETING 2.5.2 CURRENT PROJECTS 2.5.3 CEO REPORT 2.5.4 OTHER NON-PROJECT WORKING GROUP DISCUSSIONS AND PRESENTATIONS 2.5.5 ACTIONS FOR AUSTRALIA 29 29 30 34 37 42 2.6 CHILD HEALTH 2.6.1 PROGRESS AT THIS MEETING 2.6.2 ACTIONS FOR AUSTRALIA 43 43 43 2.7 CLINICAL DECISION SUPPORT 2.7.1 PROGRESS AT THIS MEETING 2.7.2 CURRENT PROJECTS 2.7.3 ACTIONS FOR AUSTRALIA 44 44 44 49 2.8 CLINICAL STATEMENT 2.8.1 PROGRESS AT THIS MEETING 2.8.2 CURRENT PROJECTS 2.8.3 ACTIONS FOR AUSTRALIA 50 50 50 50 2.9 COMMUNITY BASED COLLABORATIVE CARE 2.9.1 PROGRESS AT THIS MEETING 2.9.2 CURRENT PROJECTS 2.9.3 OTHER NON-PROJECT WORKING GROUP DISCUSSIONS AND PRESENTATIONS 51 51 51 55 2.10 EDUCATION WORKING GROUP AND MARKETING COMMITTEE 2.10.1 PROGRESS AT THIS MEETING 2.10.2 CURRENT PROJECTS 2.10.3 OTHER NON-PROJECT WORKGROUP DISCUSSIONS AND PRESENTATIONS 2.10.4 ACTIONS FOR AUSTRALIA 58 58 59 61 61 2 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.11 ELECTRONIC HEALTH RECORDS 2.11.1 PROGRESS AT THIS MEETING 2.11.2 CURRENT PROJECTS 2.11.3 ACTIONS FOR AUSTRALIA 62 62 65 72 2.12 ELECTRONIC SERVICES 2.12.1 PROGRESS AT THIS MEETING 2.12.2 CURRENT PROJECTS 2.12.3 ACTIONS FOR AUSTRALIA 74 74 75 75 2.13 HEALTH CARE DEVICES 2.13.1 PROGRESS AT THIS MEETING 2.13.2 ACTIONS FOR AUSTRLIA 76 76 76 2.14 HL7 ACTIVITIES WITH OTHER SDOs 2.14.1 PROGRESS AT THIS MEETING 2.14.2 ACTIONS FOR AUSTRLIA 77 77 79 2.15 HL7 ARCHITECTURE PROGRAM AND SAIF 2.15.1 PROGRESS AT THIS MEETING 2.15.2 CURRENT PROJECTS 2.15.3 ACTIONS FOR AUSTRALIA 80 80 80 81 2.16 INTERNATIONAL COUNCIL—GENERAL SESSION 2.16.1 PROGRESS AT THIS MEETING 2.16.2 CHIEF TECHNOLOGY OFFICER (CTO) REPORT 2.16.3 TECHNICAL STEERING COMMITTEE (TSC) REPORT 2.16.4 REGIONAL REPORTS 2.16.5 HL7 AROUND THE WORLD 82 82 84 85 86 86 2.17 INTERNATIONAL COUNCIL—AFFILIATE CHAIRS SESSION 2.17.1 PROGRESS AT THIS MEETING 2.17.2 ACTIONS FOR AUSTRALIA 87 87 91 2.18 JOINT INITIATIVE COUNCIL LIAISON 2.18.1 PROGRESS AT THIS MEETING 2.18.2 ACTIONS FOR AUSTRALIA 92 92 92 2.19 MEMBERSHIP TASKFORCE 2.19.1 ACTIONS FOR AUSTRALIA 93 93 2.20 MOBILE HEALTH 2.20.1 PROGRESS AT THIS MEETING 2.20.2 CURRENT PROJECTS 2.20.3 OTHER NON-PROJECT WORKING GROUP DISCUSSIONS AND PRESENTATIONS 2.20.4 ACTIONS FOR AUSTRALIA 94 94 94 96 97 2.21 MODELLING AND METHODOLOGY 2.21.1 PROGRESS AT THIS MEETING 2.21.2 CURRENT PROJECTS 2.21.3 ACTIONS FOR AUSTRALIA 98 98 98 99 2.22 PATIENT ADMINISTRATION 2.22.1 PROGRESS AT THIS MEETING 2.22.2 CURRENT PROJECTS 2.22.3 ACTIONS FOR AUSTRLIA 100 100 100 101 2.23 PATIENT CARE 2.23.1 PROGRESS AT THIS MEETING 2.23.2 CURRENT PROJECTS 2.23.3 ACTIONS FOR AUSTRALIA 102 102 102 105 2.24 PHARMACY 2.24.1 PROGRESS AT THIS MEETING 2.24.2 ACTIONS FOR AUSTRALIA 106 106 108 3 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.25 POLICY ADVISORY COMMITTEE 2.25.1 PROGRESS AT THIS MEETING 2.25.2 ACTIONS FOR AUSTRALIA 109 109 109 2.26 SECURITY 2.26.1 PROGRESS AT THIS MEETING 2.26.2 CURRENT PROJECTS 2.26.3 OTHER NON-PROJECT WORKING GROUP DISCUSSIONS AND PRESENTATIONS 110 110 110 114 2.27 SERVICES ORIENTATED ARCHITECTURE 2.27.1 PROGRESS AT THIS MEETING 2.27.2 CURRENT PROJECTS 2.27.3 ACTIONS FOR AUSTRALIA 117 117 117 120 2.28 STRUCTURED DOCUMENTS 2.28.1 PROGRESS AT THIS MEETING 2.28.2 CURRENT PROJECTS 2.28.3 ACTIONS FOR AUSTRALIA 121 121 122 127 2.29 TEMPLATES 2.29.1 PROGRESS AT THIS MEETING 2.29.2 CURRENT PROJECTS 2.29.3 ACTIONS FOR AUSTRALIA 129 129 129 133 2.30 VOCABULARY 2.30.1 PROGRESS AT THIS MEETING 2.30.2 CURRENT PROJECTS 2.30.3 ACTIONS FOR AUSTRALIA 134 134 134 138 3. ACRONYM LIST 139 4. REPORTS FROM HL7 AFFILIATES AROUND THE WORLD 151 4.1 HL7 Argentina 151 4.2 HL7 Australia 152 4.3 HL7 Austria 152 4.4 HL7 Brazil 152 4.5 HL7 Bosnia and Herzegovina 153 4.6 HL7 Canada 153 4.7 HL7 China 154 4.8 HL7 Colombia 154 4.9 HL7 Croatia 154 4.10 HL7 Czech Republic 154 4.11 HL7 France 154 4.12 HL7 Germany 155 4.13 HL7 India 156 4.14 HL7 Italy 156 4.15 HL7 Japan 157 4.16 HL7 Korea 157 4.17 HL7 Luxembourg 157 4.18 HL7 New Zealand 157 4.19 HL7 Netherlands 158 4.20 HL7 Norway 158 4.21 HL7 Puerto Rico 159 4 Final Report HL7 Meeting—Atlanta, USA (May 2013) 4.22 HL7 Pakistan 160 4.23 HL7 Singapore 160 4.24 HL7 Spain 160 4.25 HL7 Switzerland 160 4.26 HL7 Taiwan 160 4.27 HL7 UK 160 4.28 HL7 US 161 5 Final Report HL7 Meeting—Atlanta, USA (May 2013) 1. INTRODUCTION HL7 International is a Standards Development Organisation (SDO) originating from the USA. It provides international standards for inter-system and inter-organisational messaging, decision support, clinical text documents mark-up, user interface integration, EHR/PHR systems functionality, a health data model, and message development methodology. HL7 International produces global health informatics standards through a process of collaboration that involves its local affiliate, HL7 Australia. HL7 International Working Group Meetings (WGMs) are held three times a year. These WGMs serve two important purposes: giving the HL7 International work groups a chance to meet face-to-face to work on standards, and giving attendees the opportunity to network with industry leaders from around the world. Both of these outcomes provide a valuable educational resource for the healthcare IT community. HL7 standards are the dominant health messaging standards in the USA, Canada, Germany, Holland, Finland, Japan, Korea, Taiwan, New Zealand and Australia, and are being adopted as health messaging standards by many other countries. The May 2013 HL7 International WGM was held in Atlanta, Georgia, USA, with activities scheduled over seven days. There were 370 attendees at this meeting. The main activities ran from Sunday 5 May to Friday 10 May 2013. On weekdays formal meetings were scheduled from 9:00am to 5:00pm, however as is common for HL7 meetings, some were arranged from 7:00am and ran as late as 10:00pm. Over the past 12 months HL7 has been examining its priorities and principles. This has been a period of significant change and growth that culminated in the announcement of HL7 free access to Intellectual Property (IP) at the Baltimore WGM in September 2012. This meeting announced the commencement of a project to re-image HL7 products that will be based on the Product Line and Product Family Strategy. Additionally the recently completed Tooling Strategy findings were presented to the Technical Steering Committee (TSC), and a Risk Assessment Project that will develop a formal risk assessment process for HL7 International was initiated. It should be noted that the HL7 International standards work is not structured as "Work Items" put forward to the HL7 body for approval; rather, most projects arise from the work within the many domain specific and specialist committees. The proposed projects need to be well-defined and documented, and require approval by the relevant Steering Division and the Technical Steering Committee to ensure appropriate internal (HL7) and external (international standards development organisations) harmonisation before being commenced. This report summarises the committee proceedings, work progress, issues and actions for consideration by Australia arising from this HL7 International WGM. 1.1 OBJECTIVES OF MEETING HL7 meetings are true working meetings—not conferences—with many experts and individual groups meeting to develop, discuss and improve HL7 standards, processes and implementation guides, and to determine the most effective way to meet the needs of the stakeholders—both those present at the meeting and those in the wider community of interest. Although HL7 engagement with stakeholders in other forums is also strong (through regular—often weekly—teleconferences), the ability to influence the work program, outcomes and strategic direction requires physical presence at WGMs. The overarching objectives of HL7 meetings are to benefit the Australian health system and wider community as follows: 6 Final Report HL7 Meeting—Atlanta, USA (May 2013) • improve Australian capacity to implement health informatics standards and e-health systems by expanding local knowledge and expertise based on international best practice. • promote free trade and its benefits to health ICT by lowering the cost of integrating and implementing local health information systems—many of which are imported—and by reducing the costs to Australian exporters. Both of these outcomes require Australian requirements to be embedded in global standards so that they can be adopted in Australia, rather than having different standards across domestic and international markets. • improve Australian health information systems by facilitating a standards based approach to development and implementation, and achieving interoperability between systems. Other more specific objectives for Australian engagement in international standardisation via HL7 International include the following: • Monitoring and influencing HL7’s strategic positioning as a global SDO encourages collaboration with other international and global SDOs, and assessing and contributing to the strategic positioning of its key products (HL7 V2.x, V3, CDA, EHR, etc.) so as to encompass Australia’s health information interchange and related requirements. • • Negotiate the inclusion of messaging requirements into HL7 V2.8, CDA and V3 specifications for— - patient administration; - diagnostics (pathology, radiology); and - collaborative care initiatives, such as (but not limited to) e-discharge and e-referral. Negotiating the inclusion of Australian health sector requirements in the HL7 Standards to ensure that Australian EHR developments are supported by the upcoming HL7 and related ISO EHR Standards. • To achieve the long-term goal of a unified set of progressive inter-SDO e-health global health informatics standards, Australia is negotiating the harmonisation of ISO, HL7, CEN and other established standards development organisations (SDOs). • Monitoring and influencing new initiatives for standardising clinical data content improves Australia’s ability to unambiguously and safely exchange semantically interoperable clinical data. • Australia’s national direction setting requires assessment and influence of HL7’s work on service oriented architectures (SOA), and negotiation of the inclusion of Australian health sector requirements (in particular, those described by NEHTA) into service specifications being jointly developed by HL7. • Assessing and influencing the positioning, development, implementation, utility and effectiveness of CDA (including CDA Release 3) supports Australia’s interest in CDA and the national eHealth program. • Assessing, exploring and proposing approaches to the embedding and transportation of archetypes in HL7 V2.x messages for referral, diagnostic results and collaborative care supports Australian interest in the use of archetypes for the exchange of clinical information. • Progression of the international harmonisation of common data types and vocabulary for healthcare information will meet Australia’s identified requirements. Additional Australian interests may be pursued opportunistically as and where formally agreed upon by the community, Standards Australia and the Department of Health and Ageing (DoHA). Additional specific objectives may arise occasionally as a result of the development of Australia’s national eHealth agenda and other national interests. 7 Final Report HL7 Meeting—Atlanta, USA (May 2013) 1.2 RELEVANCE TO NEHTA PROGRAMS NEHTA has endorsed a range of Australian Standards derived from international standards work by including them in the National e-Health Standards Catalogue. As the implementation of NEHTA’s domain specific initiatives are based on many of these standards, it is important that Australia continues to be involved in the international forums that develop, manage and maintain these, and other potentially relevant health informatics standards. 1.3 MEETING LOGISTICS The table below shows the meeting schedule for all of the meeting groups. Most USA based meetings have more than 60 separate working groups and committee meetings. In addition to the working groups listed, members also attended tutorials and project specific workshops. The Australian delegation is denoted as per the following table: RDH – Richard Dixon Hughes ZM – Dr Zoran Milosevic SC – Dr Stephen Chu TW – Dr Trish Williams DH – Daniel Henzi NW – Nat Wong AM – Amy Mayer Working Group Sat Sun Mon Affiliate Agreement Taskforce (AATF) RDH Affiliate Due Diligence Committee RDH Tue Wed Thu Fri RDH Ambassador Program Anatomic Pathology Anaesthesia Architecture Review Board ZM ZM Arden Syntax Attachments Board of Directors’ Meeting RDH Clinical Context Object Clinical Decision Support AM AM Clinical Genomics 8 Final Report HL7 Meeting—Atlanta, USA (May 2013) Working Group Sat Sun Mon Tue Wed Thu Fri Clinical Interoperability Council Clinical Statement SC Co-Chair Information Session RDH TW Community Based Collaborative Care TW TW Conformance and Guidance for Implementation/Testing DICOM WG-10 Education AM Electronic Health Records RDH AM RDH Electronic Services RDH NW Emergency Care FHIR Connectathon FHIR Project NW NW NW Financial Management Foundation Taskforce Fresh Look Taskforce Governance and Operations GS1 Education Session HL7 Activities with Other SDOs RDH TW HL7 Product Line Architecture Health Care Devices TW Imaging Integration Implementation Technology Specification Infrastructure and Messaging International Council – General Session AM NW RDH 9 Final Report HL7 Meeting—Atlanta, USA (May 2013) Working Group Sat Sun Mon Tue Wed Thu Fri SC TW International Council – Affiliate Chairs RDH International Mentoring Committee ISO TC215 WG-2 ISO TC215 + IEC SC62A JWG7 RDH Joint Initiative Council (JIC) Liaison RDH Membership Taskforce (MTF) Marketing Council Mobile Health SC TW TW TW Modelling and Methodology NW Orders and Observations Patient Administration NW Patient Care SC NW NW SC SC Patient Safety Pharmacy SC SC Physician’s Meeting Policy Advisory Committee RDH Process Improvement Committee Project Services Public Health Emergency Response Publishing Regulated Clinical Research Information Management RIM Based Application Architecture SAIF Architecture Program Update Security ZM TW TW TW TW 10 Final Report HL7 Meeting—Atlanta, USA (May 2013) Working Group Sat Sun Services Oriented Architecture Mon Tue Wed ZM ZM TW Steering Divisions – Domain Experts, Foundations and Technology, Structure and Semantic Design, Technical and Support Services Structured Documents Thu Fri ZM ZM TW ZM AM SC RDH ZM ZM Technical Steering Committee Templates RDH Tooling Vocabulary AM AM AM AM AM Tutorials are also offered and are of great value to newcomers and older hands alike, and help attendees with generic changes made that may not be discussed in their individual committee areas. At this meeting 41 tutorial sessions were held concurrently with 40 work group and 9 taskforce meetings. Additionally, there were meetings for the Ambassador Program, Co-Chairs, Board of Directors, First Time Attendees, GS1 Education session, HL7 Product Line Architecture Program, CIMI and Nurses, and HL7 activities with other SDOs. The large number of concurrent sessions makes it difficult for a small delegation to effectively follow all the issues and influence change in all areas of interest to Australia. It is noted that delegates self-funded or funded by their employer to go to international meetings have no obligation to work with or relate information back to the Australian delegation, although some have done so in the past. It is clearly desirable that there be a cohesive Australian position. Given the participatory natures of the HL7 committee work it is vital that Australians are present and participate. Intensive work is done in the committees, and often more than one Australian subject matter expert is required to integrate Australian requirements into the consensus based processes. In most cases preparation beforehand of ‘Australian positions’ on the matters to be worked on is not effective, as the discussions and views often substantially change during the consensus building process. Most of the work done in committee is ‘leading edge’ standards development work that often cannot be locally previewed, assessed or commented on beforehand. As a result the selection process of the funded participants focuses on their expertise and interests, as well as their ability to effectively communicate complex technical issues and achieve the desired outcomes for Australia in a collaborative, consensus-based committee environment. As is customary, the Australian participants met on a daily basis to plan and monitor their involvement, identify any additional sessions or activities that should be covered, and identify emerging issues— particularly those that are relevant to Standards Australia IT-014 and NEHTA work plans. Australian participants also coordinate their activities through Skype. 11 Final Report HL7 Meeting—Atlanta, USA (May 2013) 1.4 RECOMMENDATIONS ARISING FROM THE MEETING Topic Issue/Action/Recommendations for Australia Recommended for Action by Architecture Review Issue: Business Architecture Model (BAM) is optimising HL7 processes to meet Product Line and Product Family expectations. NEHTA Board (ARB): Business Architecture Action: Evaluate BAM model to ensure use cases for HL7 products meet Australian expectations. IT-014 Issue: Infobutton standards address relatively simple interactions with EHR based applications, and address both patient and provider knowledge needs. US implementation is required for Meaningful Use 2. These needs are universal, and the standards offer benefits to Australian healthcare providers, consumers and vendors. IT-014-13 Model (BAM) Clinical Decision Support: Infobutton Action: Ongoing development of Infobutton standards in the Australian context to be informed by international developments. Clinical Decision Support: Virtual Medical Record (vMR) for Clinical Decision Issue: Standards Australia’s CDS project HL7 V2 Virtual Medical Record Profile is feeding into HL7 International’s CDS and V2 work and vice versa. IT-014-13 Action: International development in the vMR work and IGs to continue to inform HL7 V2 Virtual Medical Record Profile project in Australia. Support Clinical Decision Support: Virtual Medical Record (vMR) for Issue: Incorporation of nutrition and diet specifications is included in the vMR from the Diet Order DAM. Such requirements are important for decision support and patient safety for dieticians, speech pathologists, physicians, nurses and their patients in Australia. Support Action: It will be important for take up in the Australian context that these specifications for decision support are included and modelled on international Diet Order specifications. Other allied health and ancillary provider requirements should also be explored for decision support. Child Health: Issue: These potential projects are relevant to Australia’s PCEHR Child eHealth Record. Clinical Decision EPSDT Milestone and Anthropometrics Template Clinical Statement: OWL-based HL7 v3 model-model validator Action: The Australia e-Health community, specifically IT-014-06-06, IT-014-13 and NEHTA, should observe these closely, and provide input to the development of these templates. Issue: This tool may be useful for validating the conformance of patterns of detailed clinical models to CDA, as well as Pharmacy and Patient Care RMIMs. Action: It will be useful for IT14-06 subcommittees, NEHTA and PCEHR groups to keep a close watch on the development and maturation of this OWL based tool, and to evaluate its appropriateness and adequacy for adoption. IT-014-13 Allied Health Professions Australia (AHPA) IT-014-06-06 IT-014-13 NEHTA IT-014-06 and its subcommittees NEHTA Australia eHealth communities 12 Final Report HL7 Meeting—Atlanta, USA (May 2013) Topic Issue/Action/Recommendations for Australia Recommended for Action by Clinical Decision Issue: A relaxation of the amount of content of HL7 International Intellectual Property included in Standards Australia Implementation Guides may be required for this project, or in the absence of such a relaxation a review of the manner in which this Australian Implementation Guide will be structured. Standards Australia Support: VMR v2 Implementation Guide Action: Review proportion of original HL7 content in CDS implementation guides. Community Based Issue: Increased involvement of Australia in input/feedback on HCS as a foundation for information sensitivity labelling and secure handling. Collaborative Care (CBCC)/Security: Healthcare Classification Scheme HL7 Australia SA IT-014-04 Action: Notification by SA to IT-014-04 balloting of HCS, and IT-014-04 to review and comment on this to provide feedback to HL7 Australia on next ballot. (HCS) Education: Certification Project Education: e-learning Plan Issue: There is agreed and inherent value in building varying levels of certification for an advanced practitioner level as distinct from base level certification. Previously a certification project was active with the Education Work Group, however the project leader was no longer available and the project is no longer active. HL7 Australia Action: HL7 Australia to consider taking up leadership of this project to reinstate as an active project. WGMs Issue: An e-learning course for Australia could be established. The course can be set up for the HL7 Australia Board to review. (This issue was reported by Heather Grain at the Phoenix 2013 WGM and continues to be relevant.) Future Education representative delegations to HL7 Australia Education Council Action: HL7 Australia Education Council to review e-learning course materials. Electronic Services/ T3SD EHR WG Re-ballot of R2 EHR-S FM Issue: Work in Australia on update of its electronic services may be complementary to other HL7 work. HL7 Australia Action: Keep the Technical and Support Services Steering Division updated with the progression of the ‘Affiliate in a Box’ concept being developed by HL7 Australia. Issue: There is a mismatch between the HL7 and ISO re-ballot requirements in relation to the next round of normative balloting to finalise the ISO/HL7 10781 EHR-S FM R2 standard. The leaders of EHR WG have sought advice and assistance on managing the potential issues through the JIC Chair (Richard Dixon Hughes), the ISO/TC 215 Secretariat (Lisa Spellman) and the ISO/TC 215/WG1 Secretariat (Naomi Ryan). ISO/TC 215 WG 1 Secretariat (Naomi Ryan) Richard Dixon Hughes Action: Naomi Ryan (WG1) and Richard Dixon Hughes to consider issues relating to harmonisation of DIS2 ballot of ISO/HL7 10781 with HL7 N3, and assist HL7 EHR WG Co-Chairs and ISO/TC215 secretariat in finalising a joint publication in the most effective manner. 13 Final Report HL7 Meeting—Atlanta, USA (May 2013) Topic Issue/Action/Recommendations for Australia Recommended for Action by EHR WG: Issue: An Australian review and response to these two ballots of the ISO/HL7 PHR-S FM will be required in coming months. This was previously covered by IT-014-09, but now falls under IT-014-13 with its broader responsibility of ‘Clinical workflow and care systems’. The HL7/N2 ballot through HL7 Australia is likely to close much earlier than the ISO/DIS2 ballot. A consistent approach is desirable. IT-014 HL7/N2 and ISO/DIS2 ballot of PHR-S FM HL7 Australia Action: IT-014-13 and former members of IT-014-09 to be invited to prepare ballot responses to HL7/N2 and ISO/DIS2 ballots in collaboration with HL7 Australia. EHR WG: Proposals for R3 of EHR-S FM Issue: While preparation of Release 3 of the EHR-S FM is still some time away, the preparation of the PSS for this work is already being progressed by Functional Information Model (FIM) Sub-Group, who are recommending a fundamental re-engineering based on incorporation of their current approaches. Although this may or may not have merit, the preliminary work on the FIM is strongly US based and there is a risk that the International applicability of the EHR-S FM as an international standard will be prejudiced or lost in this process. It is desirable that this move be closely monitored and if necessary addressed at an early stage to ensure that any outcomes are internationally applicable. IT-014 HL7 Australia Australian delegations to HL7 WGMs Action: Future delegations, IT-014 and HL7 Australia monitor the proposals and scope statement for R3 of the EHR-S FM to ensure that future revisions are likely to remain relevant as an international standard. As the HL7 process for approval of Project Scope Statements is an internal function of the TSC without external input, raising early harmonisation of this work through JIC should be considered. EHR WG: Usability Sub-Group Issue: Participation in the work of the new Usability Sub-Group of the EHR WG by Australians with interest and expertise in usability of systems would be welcome. The project is commencing with a literature review and white paper, with a view to identifying relevant usability criteria and incorporating them into the EHR-S FM. Australian and related ISO/TC 215 guideline documents on user interfaces and the application of clinical decision support are among the key references identified to date. IT-014 HL7 Australia Action: IT-014 and HL7 Australia to circulate information on the HL7 EHR Usability Project among relevant experts with an invitation for them to participate in the activities of the Usability Sub-Group of the EHR WG. Health Care Devices: Ventilator Nomenclature Issue: Standardisation of measurement points and variables. Action: Advise Standards Australia Health Care Devices mirror group HE-003 of revisions and canvas for potential members or manufacturers to engage in this work. Standards Australia (HE-003) 14 Final Report HL7 Meeting—Atlanta, USA (May 2013) Topic Issue/Action/Recommendations for Australia Recommended for Action by HL7 Board: Issue: Changes to the HL7 membership model, membership benefits, education and certification services, and IP licensing policies have potential consequences for the HL7 affiliates, including HL7 Australia. Although generally positive for worldwide adoption and acceptance of HL7 standards, these changes may impact the perceived benefit of being an HL7 Australia member and its contractual relationships. HL7 Australia Changes to HL7 International membership model, services and licensing policies HL7 Board: IP Licensing Taskforce Action: HL7 Australia to continue monitoring, analysing and responding to the emerging changes in the HL7 International membership model, services and IP licensing policies. Issue: HL7 international has formed an IP licensing taskforce to provide advice through the Membership Committee on IP licensing issues that arise from free licensing of HL7 standards. The IP licensing taskforce also takes note of potential interactions with the new membership model, including possible difficulties in the different treatments accorded to ‘derivative works’ under various contractual arrangements. Richard Dixon Hughes has been appointed to the taskforce based on his legal qualifications, his current role as Chair of HL7 Australia, and his knowledge of agreements between Standards Australia and HL7 Australia in relation to the use of HL7 International IP in Australian Standards and lower consensus publications. Chair of HL7 Australia. Action: Richard Dixon Hughes to participate actively as an appointed member of the HL7 International IP Licensing Taskforce, with a view to ensuring consistency between the various types of IP licenses, the affiliate agreement and other contractual arrangements for use of HL7 International’s intellectual property. HL7 Terminology Authority (HTA) Issue: The HL7 Board has accepted a recommendation to form an HL7 Terminology Authority (HTA) under the agreement between IHTSDO and HL7 International to act as a gatekeeper to ensure that content from HL7 is submitted to IHTSDO in a controlled manner. Of the five members of the Authority, two must come from within the HL7 affiliates, be appropriately qualified and experienced, and be prepared to participate regularly in required teleconference calls. A call for nominations is to be issued. Appropriate Australian candidates should be given the opportunity to nominate. HL7 Australia Standards Australia Action: The HL7 call for nominations of suitably qualified persons to serve on the HTA to be circulated to IT-014-02, IT-014-06 and NEHTA (Terminology Services). International Council: Staging of IHIC 2013 in Sydney Issue: HL7 Australia is organising the International HL7 Interoperability Conference (IHIC 2013) in Sydney in a time slot adjacent to the ISO/TC215 meeting being planned for October 2013, and has received $US5 000 support toward this end from the HL7 International Council. There is a lot still to be organised. HL7 Australia Action: HL7 Australia to progress the organisation of IHIC 2013 in Australia in October 2013 and get calls for papers and publicity in progress by end of June 2013. 15 Final Report HL7 Meeting—Atlanta, USA (May 2013) Topic Issue/Action/Recommendations for Australia Recommended for Action by HL7 Architecture Issue: It is not clear at present how Product Line/Product Family concepts from the ARB BAM project are related to the SAIF Architecture Program. HL7 Australia Program and SAIF: SAIF Artifact Definition Mobile Health: Action: Contribute to and influence product line/product family developments while linking it with the experience and deliverables from the SAIF Artifact Definition project. Issue: Benefits and opportunities for mobile health include extending provider mobility especially for community clinics, rural and remote and more mobile workforces (e.g. district nurses), improving safety through identification standards, record keeping, and identification of devices for supply chain management IT-014 Standards Australia Future delegations Action: Integrate mobile health within Australian context and work programs. Modelling and Methodology & Patient Administration: FHIR Resources Patient Care: Allergy/Intolerance and Adverse Reaction, Care Plan DAMs; CCS FM; and FHIR resources models Pharmacy: HL7/IHE Medication Profile Issue: FHIR Resource development requires ongoing Australian input to influence the inclusion of data elements that would be considered core for Australia Action: Continue to support and engage Australian subject matter experts in the development of domain specific resources, including Encounter resource for community contexts. Issue: Ongoing work on allergy/intolerance and adverse reaction in Care Plan DAMs needs to be noted by relevant IT-014 groups and considered for incorporation into relevant Australian activities. NEHTA Standards Australia Future HL7 delegations IT-014-06-04 IT-014-06-05 Action: Allergy/Intolerance, Adverse Reaction, and FHIR resource Models are of interest to and should be monitored by IT-014-06-04, IT-014-06-05, IT-014-06-06 and IT-014-13. IT-014-13 Care Plan and CCS are of interest to IT-014-06-06, IT-014-09 and IT-014-13. Australian eHealth IT-014-06-06 All these projects are of interest to NEHTA and PCEHR projects. Communities Issue: The medication profile work is of high importance to Australia. IT- 014-06-04; IT-014-06-06 and IT-014-13, NEHTA and PCEHR projects in particular should monitor and provide relevant input to this activity. IT-014-06-04 IT-014-06-06 IT-014-13 projects Action: The ISO WG6 activities remain a concern to other international groups. There needs to be a closer link between the Australian delegate to ISO WG6 and IT-014-x groups to ensure visibility of the ISO projects. Australian eHealth Security: Issue: The development of the Security implementation guides needs wider than US input. HL7 Australia ISO/TC215 WG 6 HL7 DS4P IG Action: It is important that Australia contribute to the development of this project to ensure its companion work is suitable for Australia in the future. communities IT-014-04 16 Final Report HL7 Meeting—Atlanta, USA (May 2013) Topic Issue/Action/Recommendations for Australia Recommended for Action by SOA: Issue: The OMG MDMI standard can support many types of information mappings but is not sufficient to support general behavioural mapping. IT-014-09 Cross Platform Interoperability Implementation Guide for SOA Ontology WG Action: Continue analysis of alternative approaches to support behavioural mapping, including the use of an MDMI standard and other MDHT components as well as new SOA Ontology concepts. Immunisation SOA: SOA Service Ontology Issue: The ontology paper included on the SOA ontology wiki page (http://hsspinfrastructure.wikispaces.com/HSSP+SOA+Service+Ontology) provides the basis for the development of a rich set of discoverable and interactive e-Health services. When balloted it needs to be explained, discussed, socialised and commented upon by a wide audience within the health standards and software community. HL7 Australia IT-014-09 NEHTA Action: Develop and present a brief paper for Standards Australia that outlines the approach, and consider the application of SOA ontology for Australian e-Health (e.g. National Health Services Directory, the ELS and PCEHR). Note that the SOA Ontology is directly related to the e-Health Interoperability Framework developed within IT-014-09, and thus this group is a natural home to progress this work. Action: Communicate SOA Ontology work to other relevant organisations and encourage the use of the ontology in SOA oriented solutions. SOA: Patient Care Services Issue: This seems to be well-defined project and the only issue would be feasibility of executing it in sync with other projects of the SOA or other WGs. IT-014-09 Future delegates to Co-ordination Project Action: Investigate how the PCC service can be expressed using the concepts proposed by the SOA Ontology project. SOA Ontology WG SOA/Patient Care: The SOA/Patient Care ordering services interface specification project is relevant and of interest across the IT-014 and the wider eHealth standards community. IT-014-06-04 Ordering Services Interface Specification Action: SOA/PC work on ordering services interface specifications to be reviewed by IT-014-06-04, IT-014-06-05 and IT-014-13 as basis for wider discussion and need for complementary Australian activity. IT-014-06-05 IT-014-13 Australian eHealth communities Structured Documents: CDA R3 Issue: CDA R3 will be submitted for ballot in September 2013. Action: Monitor new CDA R3 developments, and understand industry acceptance and impact on Australian specifications that are based on R2. NEHTA and IT-014 committees are to monitor the progress on this item. NEHTA IT-014 HL7 Australia Also monitor interplay of R3 with FHIR specification that will be published in the same balloting period. 17 Final Report HL7 Meeting—Atlanta, USA (May 2013) Topic Issue/Action/Recommendations for Australia Recommended for Action by Structured Issue: The Patient Generated Document (PGD) IG is intended to provide implementation guidance on the CDA header and body elements for documents authored by patients or representatives of patients. The PGD header now includes international inputs based on Australian work to align better with international requirements. It is recommended that Australia be further engaged in the development of PGD body components. NEHTA Documents: Patient Authored Documents IT-014 Action: NEHTA and Australia should participate and include Australian patient generated documents body elements to this CDAIG on PGD IG. This will enable Australian implementers and standards bodies to contribute and align with international standards for patient generated documents. Structured Documents: Questionnaire and Questionnaire Response CDA Implementation Guides Structured Documents: Automation and open source tooling for clinical information modelling Structured Documents: Clinical Oncology Treatment Plan and Issue: Electronic interchange of meaningful question and response documents between the practitioner and the patient. NEHTA Action: NEHTA and Australia should actively participate in the standardisation of questionnaire and questionnaire response documents. The Australian requirements for Consumer Entered Health Assessment and Bluebook Questionnaire requirements should be augmented to the universal realm. This is to align Australian consumer/healthcare provider questionnaire documents with the international questionnaire framework. IT-014 Issue: There is a need for formal methodology or best practices for developing CDA templates and implementation guides, and appropriate tooling to support repeatable processes and deal with the proliferation of templates. NEHTA Action: Investigate the use of Model Driven Health Tools (MDHT) as a way of providing programmatic access to the information model associated with templates, and supporting a scalable and repeatable approach to the clinical information modelling. Consider positive experience reported at this WG meeting regarding harmonisation between C-CDA and IHE and as a way of dealing with the proliferation of templates. Issue: The current C-CDA Implementation Guide specification is a good document from both the modelling and clinical perspective, but is developed for the US Realm. Action: Consider this document for relevant future developments related to Australian e-health clinical oncology specifications. IT-014-06-04 IT-014-06-06 IT-014-13 DoHA Summary HL7 Australia Vocabulary: Issue: HL7 Vocabulary Working Group requires a deep understanding of the various technical vocabularies and project bases. At the current meeting the Vocabulary tutorial was cancelled and therefore mentored delegate was unable to fully engage in Vocabulary WG development. DoHA IT-014 Action: Consider Australian vocabulary priorities and potential for development and support of Australian subject matter experts in future delegations. 18 Final Report HL7 Meeting—Atlanta, USA (May 2013) Topic Issue/Action/Recommendations for Australia Recommended for Action by Vocabulary: Issue: The use of HingX as a trial needs to be noted by Standards Australia, for consideration of adoption of the tool as: Future HL7 HingX 1. 2. It may be useful to Standards Australia as a platform for collaboration; and The structure of resources needs to be well defined and input to the structure required. Delegation (to review progress of this project) Action: This action is carried over from a recommendation made by Heather Grain in the 2013 Phoenix Delegation Report. Please monitored by future delegations for information to support Standards Australia's review of tool and opportunities for use. 19 Final Report HL7 Meeting—Atlanta, USA (May 2013) 1.5 FUNDING SOURCES SUMMARY AND AUSTRALIAN ATTENDANCE Twelve Australians attended as representatives for the duration of this HL7 meeting, six of whom were in the formal ‘delegation’. The funding source for these delegate numbers is indicated in the table below. DoHA provided funding assistance for the following delegates: • Daniel Henzi • Richard Dixon Hughes • Amy Mayer • Dr Zoran Milosevic • Dr Trish Williams • Nat Wong The following delegates attended on behalf of NEHTA: • Dr Stephen Chu Funding Source Number Change from Previous meeting Full funding by employer: Private 0 0 Full funding by employer: States/Territories or National Initiatives (NEHTA) 1 -2 Funding assistance—DoHA through Standards Australia Funding Agreement 6 -3 Total: 7 -5 1.6 AUSTRALIAN LEADERSHIP POSITIONS The table below lists leadership positions held by Australians at the HL7 meeting in May 2013. Attendee Position Funding Source Work Group or Committee Dr Trish Williams Co-Chair Standards Australia via the DoHA Funding Agreement Security Dr Stephen Chu Co-Chair NEHTA Patient Care Nat Wong Co-Chair Standards Australia via the DoHA Funding Agreement Electronic Services Co-Chair Standards Australia via the DoHA Funding Advisory Council to the Board of HL7 International Richard Dixon Hughes 20 Final Report HL7 Meeting—Atlanta, USA (May 2013) Attendee Position Non-Voting Member Funding Source Agreement Chair—HL7 Australia Work Group or Committee HL7 International Board of Directors International Council Affiliate Chairs Meeting International Membership Affiliation Taskforce (IMATF) Dr Zoran Milosevic Invited Member Affiliate Due Diligence Committee (of HL7 International Board) Appointed Member Affiliate Agreement Taskforce (of the International Council) Invited Member Policy Committee (of HL7 International Board – invited by HL7 Chair) Appointed Member Intellectual Property Taskforce (invited by HL7 Board) Chair (2013/14) Joint Initiative Council (JIC) for Global SDO Health Informatics Standardization ArB Member Standards Australia via the DoHA Funding Agreement Architecture Review Board 21 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2. 2.1 2.1.1 WORKING GROUP REPORTS ADVISORY COUNCIL PROGRESS AT THIS MEETING The Advisory Council performs most of its work through monthly teleconferences and a face-to-face meeting at the annual Board retreat, but does not meet at WGMs. As the Chair of the Advisory Council, Richard Dixon Hughes provides input on its behalf at the face-to-face Board meetings held at WGMs. In the months leading up to this WGM the Council was consulted on several occasions, and provided considerable further input on changes in HL7 membership benefits and fees following the strategic decision to license HL7 standards and selected other HL7 Intellectual Property free of charge. At this meeting Richard Dixon Hughes was asked to join a taskforce providing specialist advice to the HL7 Membership Committee on questions related to intellectual property and derivative works. 2.1.2 ACTIONS FOR AUSTRALIA No actions required at present. 22 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.2 2.2.1 AFFILIATE AGREEMENT TASKFORCE PROGRESS AT THIS MEETING The affiliate agreement, which provides a common framework for the contractual arrangements between HL7 International and its affiliates around the world, comes up for renewal every two years. At the January 2013 meeting the International Council (Affiliate Chairs) re-established the Affiliate Agreement Taskforce (AATF) with Philip Scott (UK) as Chair to identify and agree any further amendments to the standard affiliate agreement for the next renewal cycle, commencing 1 January 2014. Richard Dixon Hughes was re-appointed as a member of the re-established AATF. Working by email and teleconference over the period between the January and May WGMs, the AATF identified potential changes to the agreement. These were discussed in detail over several quarters at the May 2013 meeting, with an updated draft being prepared. The changes included the following: • A proposal for agreements to run for one year rather than two years, so as to accommodate the aspirations of some affiliates who feel that a two year period will inhibit adoption of other governance changes proposed in last year’s International Membership and Affiliation Taskforce (IMATF) report. HL7 Australia prefers two year terms. • Accommodating the terms under which HL7 is now licensing its standards and other selected intellectual property without charge, and related changes to membership models and fees. • Better integration with the HL7 International charter and by-laws as the source of member classes and governance rights. • Anticipating an expansion of HL7 direct education and certification activities. • Many further refinements to improve the clarity and legal precision of a document that originally grew from being more of a policy statement than a contractual agreement. In total some 35 changes were agreed. A revised version 0.4 has been prepared and circulated for AATF comment before being opened up for input from the International Council and feedback from HL7 Headquarters staff. Richard Dixon Hughes has suggested three further refinements to version 0.4, which have been reviewed and recommended for acceptance by the AATF Chair. The next steps will be to provide the draft to the full International Council and HL7 International HQ for further review, comment and agreement in principle. Following a resolution of any further issues, it will then be submitted to the HL7 Board for approval and distributed to affiliates for execution. 23 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.2.2 ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by Affiliate Agreement Issue: The AATF is producing the next version of the affiliate agreement between HL7 International and its affiliates (including HL7 Australia) to commence in January 2014. It is important that the agreement continues to facilitate HL7 Australia's arrangements with Standards Australia for the production and publication of Australian HL7 implementation guides as Australian Standards and lowerconsensus publications. HL7 Australia Taskforce (AATF): Renewal of affiliate agreement 2014–2015 Richard Dixon Hughes Action: HL7 Australia to continue monitoring and negotiating the affiliate agreement for 2014 with the aim of ensuring that it enables the continued availability of HL7 materials in Australia under reasonable commercial terms sufficient to allow the continued publication of Australian HL7 Implementation Guides. 24 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.3 2.3.1 AFFILIATE DUE DILIGENCE COMMITTEE PROGRESS AT THIS MEETING The Affiliate Due Diligence Committee (ADDC) assists HL7 International by reviewing applications to establish an HL7 affiliate. This includes receiving and considering applications, conducting enquiries, and making recommendations to the HL7 Executive Committee, who then forward their recommendation on to the Board of Directors for final approval. The ADDC is also responsible for assisting HL7 International maintain the affiliation criteria and associated processes, and providing information and guidance for organisations considering becoming the HL7 affiliate in their country. In addition to face-to-face meetings at WGMs, the ADDC has monthly conference calls and may meet more frequently when required to progress particular matters. Richard Dixon Hughes is a member of the ADDC. Beat Heggli (Chair of HL7 Switzerland) took over as Chair of the ADDC when Catherine Chronaki stepped down shortly after her term on the Board of HL7 International expired at the end of December 2012. The following were among the matters addressed when the ADDC met at this WGM: • An application has been lodged to form HL7 Malaysia. The report prepared by Richard Dixon Hughes following his interview of Dr Khadzir (Head of the Health Informatics Centre and Deputy Director, Planning and Development Division, Ministry of Health Malaysia) was discussed. It was noted that the application from Malaysia was of generally good quality, the petitioners represented substantial and balanced interests, and that misunderstandings over the government representation had been addressed fully in the report. It was resolved to recommend progression of the HL7 Malaysia application to the Executive Committee (EC). [EC agreement and subsequent steps were successfully completed in time for a Board approval at its June meeting, with HL7 Malaysia then being invited to complete the affiliate agreement and other formalities of becoming an affiliate.] • The status of the application from the Philippines was addressed. Concerns over the status and breadth of representation of the various petitioners were discussed. It was noted that their affiliations could be interpreted in different ways, and that when the principal function of each petitioner’s employer was taken into account, a more than appropriate balance was evident. Diego Kaminker undertook to complete the interview process, with support from Richard Dixon Hughes if required. The processing of this application is taking too long to progress. • The status of other applications was discussed. Substantial progress has yet to be achieved on the foreshadowed applications from Denmark, Poland and Slovenia. Follow-up actions were assigned to European members of the ADDC. It was noted that free of charge licensing of intellectual property in HL7 standards would increase the potential for official sanction of HL7 standards but may reduce the short-term incentive to form affiliate organisations. • Time slots for regular conference calls in the European evening toward the end of the month were discussed. These will be confirmed by a doodle poll. • A new ADDC member to replace Supten (whose term ended in December 2012) was discussed. Supten did not wish to be reappointed due to having taken up a new leadership position in the national eHealth program and retiring from being Chair of HL7 India. Several replacement candidates were identified and will be sounded out. 2.3.2 ACTIONS FOR AUSTRALIA No actions required at present. 25 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.4 ARCHITECTURE REVIEW BOARD 2.4.1 PROGRESS AT THIS MEETING Key topics addressed at this meeting were: • the completion of the SAIF CD and a presentation of Healthcare SOA Ontology (project in the SOA WG) as a SAIF Implementation Guide for services; • Business Architecture Model (BAM) updates and communication to SD WG; and • administrative (i.e. the revision and revitalisation of the ARB membership) and planning issues. In terms of planning it was agreed to assign specific future areas of interest (e.g. W3C semantic web and FHIR) to specific members of ARB, who would report regularly on developments in these areas. 2.4.2 CURRENT PROJECTS The following projects are reported on in this section: • Project #365: Service Aware Interoperability Framework (SAIF); and • Project #915: Business Architecture Model (BAM). 2.4.2.1 PROJECT #365: SERVICE AWARE INTEROPERABILITY FRAMEWORK PROJECT OBJECTIVE/TOPIC SUMMARY SAIF provides HL7 with an Interoperability Framework (i.e. a set of constructs, best practices, processes, etc.) that enable HL7 specifications to achieve cross-specification consistency and coherency irrespective of the chosen interoperability paradigm (messages, documents or services). SAIF consists of four core ‘frameworks’ including— (1) information (including RIM, data types, vocabulary bindings, etc.); (2) behaviour (subsuming the existing Dynamic Model); (3) enterprise conformance and compliance (including HL7’s existing Implementation and Conformance standards); and (4) governance. Originally titled SAEAF (Services Aware Enterprise Architecture Framework), the name caused confusion with other enterprise architectures such as Zachman and Togaf. It was renamed in February 2010. SAIF should be regarded as an adjunct to any EAF that focuses on Working Interoperability (WI). It is a framework that brings from SOA practice two critical constructs which significantly enhance the path to WI, for example— • the separation of concerns (static vs. behavioural semantics); and • the formal notion of contracts SAIF is Interoperability-Paradigm-neutral (i.e. it is equally applicable to projects seeking to develop solutions that use documents, messages and/or services). 26 Final Report HL7 Meeting—Atlanta, USA (May 2013) PROJECT ACTIVITY/ISSUES AT THIS MEETING At this meeting Zoran Milosevic introduced the ARB to a new project that can be regarded as a SAIF Implementation Guide for eHealth services. This is the HL7 SOA Healthcare Ontology Services Release 1, which was submitted as a ballot for comment in the January–May 2013 cycle. This project uses SAIF’s behavioural and governance framework as a basis for a conceptual model for understanding, using, constructing, composing, managing and evolving eHealth services. The ARB sees this work as a good starting point for consistent description of behaviour related artefacts within HL7 and informing related BAM aspects, and as a valuable link between different projects of the HL7 SOA WG. The relationship with the HL7 SOA WG also serves as an indirect link to the Object Management Group. It was also noted there is an outstanding item where current SAIF CD document needs to be completed with the Glossary attachment, and this is the work that has been finalised in September 2012 through joint work of Cecil Lynch and Zoran Milosevic. A protégé based description of the Glossary was submitted to the ARB, but due to an administrative oversight this has not been included in the SAIF CD draft and will be addressed in the next cycle. 2.4.2.2 PROJECT #915: BUSINESS ARCHITECTURE MODEL PROJECT OBJECTIVE/TOPIC SUMMARY HL7 proposes to change their organisational structure, process and policies to become more product line focused and customer focused. However HL7 currently does not have an updated, detailed, comprehensive and consolidated description of its current organisational structures, roles, processes and policies other than the Governance and Operations Manual (GOM). Further, HL7 does not have a detailed and documented description of its ideal goal organisational structures, roles, processes and policies other than the GOM. To address these issues HL7 intends to develop a business architecture model (BAM). The purpose of this document is to define an architecture methodology including guidelines, meta-models and work phases with inputs, steps and outputs for the development of the BAM. PROJECT ACTIVITY/ISSUES AT THIS MEETING It was agreed that product family and product line definitions need to be further clarified, possibly defining the attributes of each so as to support a downstream automated search. It was also agreed that the spread sheet based approach to capture BAM processes, artefacts and roles is cumbersome to maintain so a more formal and visually friendly approach should be adopted. The use of UML activity diagrams will be pursued from now on and an initial version was worked on during the meeting. Note that the key distinction between product lines and product families was communicated to the HL7 community via the May 2013 HL7 newsletter (published at http://www.hl7.org/documentcenter/public_temp_4F3763D7-1C23-BA170C1A04FF83EFF235/newsletters/HL7_NEWS_20130506.pdf). Finally the ARB has presented the key elements of the new BAM methodology to the TSC as well as to the SD WG, which is an early adopter of the BAM methodology. The SD WG will be applying the BAM governance, management and methodology to each of the product family and product lines. 27 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.4.3 ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by Architecture Review Issue: Business Architecture Model (BAM) is optimising HL7 processes to meet Product Line and Product Family expectations. NEHTA Board (ARB): Business Architecture Action: Evaluate the BAM model to ensure use cases for HL7 products meet Australian expectations. IT-014 Model (BAM) 28 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.5 2.5.1 HL7 BOARD PROGRESS AT THIS MEETING The Board of HL7 International met for the first three quarters on Tuesday 7 May. One of the major topics permeating discussions at all HL7 Board meetings continues to be the policy implications and actions flowing from HL7 international standards and selected IP being licensed at no cost from 1 April 2013. At this Board meeting gave particular attention to the— • progress of the recently formed Membership Committee, which replaced the former Membership Taskforce (MTF) in recommending potential changes to HL7 International's membership model including possible changes in membership levels, benefits and fees; • extent of the other intellectual property which would be freely available under licence; and • what level of restrictions might be appropriate to the creation, publication or distribution of ‘derivative works’ and the re-publication or distribution of the standards in other forms. Among the other significant matters considered by the HL7 Board at this meeting and reported later in this section are the— • formation of HL7 Terminology Authority to enable HL7 International to moderate and register SNOMED CT concepts under IHTSDO rules; • location and budgetary impact of May 2015 WGM, proposed to be held in France; • Treasurer’s report; • CEO’s report (including updates on other appointments to the senior executive team); and • introduction and presentation by the new member of the HL7 International staff—Ticia Gerber, Director of Global Partnerships and Policy. As the details of some matters are still under consideration by the HL7 Board, this report is primarily based on information that is either publicly available, or has been presented or discussed in other open forums (including material presented in the General Sessions at the WGM). From an organisational (as distinct from a technical perspective) HL7 strategic activities are largely being progressed within the following areas: • Membership – where much of the activity is focussed on the work of the Membership Committee, whose recommendations provide the basis for Board consideration of changes to the membership benefits and dues structures, including a range of new initiatives as outlined in section 2.5.2.1. • Education—Now headed by Sharon Chaplock, this division is looking at new approaches to deliver new coursework and utilise new technology. The HL7 Education Portal will be available in June 2013. • Partnership and Policy—HL7 has just appointed Ticia Gerber to head this division. Currently the focus is on global benefactors and funding opportunities, and the increasing role of HL7 policy issues on the WGs and committees. • Marketing and Communications—Carol Carmick head this division and is investigating new global marketing initiatives by gathering data and analysis on new branding and rebranding efforts. • Communications and Outreach—Andrea Ribbick has been active in media and PR. Andrea is responsible for internal and external messaging, and has facilitated the new monthly news brief. 29 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.5.2 CURRENT PROJECTS 2.5.2.1 PROJECT: UPDATE OF HL7 MEMBERSHIP STRUCTURE AND BENEFITS PROJECT OBJECTIVE This project relates to the activities of the Membership Committee (MC), which was established by the HL7 Board in light of the need for ongoing work following the implementation of its decision to licence HL7 standards and other selected IP at no cost. The mission of the Membership Committee is to advise the HL7 Board in the following three areas— 1. new membership benefits to retain current and attract new members of HL7 International; 2. models for expanding member categories, activities, meetings and conferences beyond the technical processes of standards development; and 3. a functional framework for the relationship of the affiliates to HL7 International. The project includes recommending coordinated changes to HL7 International’s membership structure, benefits packages, other services, by-laws, IP licences and support infrastructure to implement the proposed changes in ways that increase member engagement and protect the viability of HL7. In this regard the Membership Committee continues the work of the former Membership Taskforce of which Richard Dixon Hughes was a member (his involvement is now reduced to participation in the IP Licensing Taskforce due to the pressure of competing commitments). The current members of the Membership Committee are Grant Wood (Chair—InterMountain Healthcare), John Hatem (Oracle), Craig Gabron (BCBS of South Carolina), Ed Hammond (Duke University), Philip Scott (HL7 UK), Diego Kaminker (HL7 Argentina), Russ Leftwich (CMIO, Tennesee Office of eHealth) and Dave Shaver (Corepoint). The work is supported by a senior strategic planning consultant, Virginia Riehl. It is understood that her engagement in this activity has overtaken and continues to be resourced through an extension of Project #766 HL7 Business Plan Contract, which was first registered in March 2011. PROJECT ACTIVITY/ISSUES AT THIS MEETING A considerable proportion of the Board’s time at this meeting was spent receiving and reviewing recommendations from the Membership Committee, with a focus on the proposed benefits structure and its relationship to the various categories of membership. The work of the committee has included the review of evaluation data collected from various sources, including surveys and questionnaires to identify potential benefits of engagement with HL7, with a strategic focus on— • repositioning HL7 as an organisation that both develops standards and supports implementations, • identifying and addressing potential areas of growth, and • sustaining and growing HL7 membership and revenue. Assessment of potential benefits Potential benefits were developed and assessed in terms of five key elements: description of benefit, administrative overhead, market assessment, implementation requirements and a final recommendation. 30 Final Report HL7 Meeting—Atlanta, USA (May 2013) The committee has both a short term and a longer term agenda. The immediate priority is those recommendations needed for HL7 to offer a revised membership package in October. Accordingly some proposed benefits were recommended for progression, others are to be the subject of more detailed research, and some have been set aside for possible further consideration down the track. The HL7 Board agreed that the following initiatives should be progressed to be ready for roll-out in September 2013— • current work on implementation tooling; • current machine-processable artefacts (subject to further TSC recommendations as to implementation approach)—the identification of those artefacts to be freely available as part of the HL7 standards and which will be restricted to a member benefit remains in contention; • small meetings with HL7 leaders and knowledge experts; • onsite instruction; and • members only webinars. The following initiatives are to be progressed for roll-out by the end of September 2013, subject to satisfactory further development and the gathering of supporting data— • HL7 Help Desk Service—subject to further market assessment to determine demand and better design the service; • User Groups—subject to further research to understand demand and determine how the benefit should be designed; and • an hour with an expert—known HL7 experts still to be surveyed in order to determine topics, availabilities and fee structures. The following initiatives continue to be progressed for potential roll-out at some future time, subject to satisfactory further development and the gathering of supporting data: • The interoperability testing service will proceed by way of an RFI to assess the scope, options, costs, revenue sharing and potential partnerships. It is proposed that this service will be restricted to CDA, V2 and V3 messages with those artefacts required for MU and attachments in the US being a possible priority. Further research on vendor requirements is required to assess testing priorities. • Further research is needed to understand the demands, the priorities, and how to deliver benefits through HL7 Connectathon. • Further market research is needed to determine what new implementation tooling to invest in. The resources, costs and timeframe needed to develop required tools will also need to be determined. • Further market research is needed to determine what new machine-processable artefacts to invest in. The resources, costs and timeframe needed to develop them will also need to be determined. The following potential initiatives have been deferred for consideration at a later time and are not being progressed further at present— • conducting a vendor showcase (potentially at WGMs); • certified HL7 educator; • trending information on who is using HL7; and • listserv to announce RFPs. 31 Final Report HL7 Meeting—Atlanta, USA (May 2013) Access to Work Group materials With access to HL7 standards becoming freely available, full participation in the development of HL7 standards is increasingly being seen as a member benefit. This raises many policy issues for the Board of HL7 International, which is trying to balance ANSI requirements for access to and involvement in the standards development process against the needs to have clearly defined member benefits and the quality of the resulting products. It had been recommended by the former Membership Taskforce that read only access to all HL7 WG listservs, wikis, agendas and minutes be openly and freely available to both members and non-members, but that the ability to write to HL7 listservs and wikis be restricted to members and to subscribers who pay (say $200) for the privilege (on a per-WG, per annum basis). On further investigation it was found that implementation of this recommendation would involve considerable re-engineering of HL7’s current listserv and wiki support platforms at an indeterminate cost. These matters were discussed at some length but not resolved entirely. The board agreed to move along the following lines— • provide open access to WG minutes and agendas; • restrict access for material under development, such as pre-balloting artefacts, standards and IGs to members—this is to reinforce the three months period for members-only access after standards and IGs are balloted; • the ability to ask questions on the listserv will be limited to members in order to be consistent with the help desk benefit; • have staff research and report options for implementing these approaches; and • survey work groups regarding the impact of maintaining two wikis so that restricted members-only materials would be behind a firewall. Impact on affiliates The following recommendations of the Membership Committee relating to arrangements with affiliates were apparently accepted by the HL7 Board but without any specific resolution— • affiliate voting rights and other benefits under the current affiliate agreement continue unchanged; and • development of new benefits by HL7 International should if possible take approaches that can be leveraged by affiliates or that leverage the work of affiliates. It is noted that achievement of these outcomes (particularly the second) requires good communication between HL7 International and the affiliates, and ongoing active participation by the affiliates in the work of the Membership Committee, in order to influence the development and specification of new benefits and the classes of participation to which they apply. Collaboration with ONC Help Desk The ONC in the United States is developing a help desk to support the progressive roll-out of legislated MU (Meaningful Use) capabilities. Given the cost of infrastructure required to run a help desk service, consideration had been given to HL7 coming to an arrangement with ONC for accessing HL7 help desk capability through the ONC help desk capabilities. Although use of the ONC facility could potentially provide a contact point for initial triage, it is not a good strategic fit with HL7’s requirements—particularly in respect of the loss of branding, reduced control over 32 Final Report HL7 Meeting—Atlanta, USA (May 2013) triage, tracking and quality, losing the perception of a member benefit and associated credit, and reduced potential for HL7 revenue generation. It was also noted that ONC would not necessarily have as broad a focus as HL7. On the other hand there would be synergies for HL7 and ONC to work together. The path to be followed is for HL7 International to develop a website presence under its own control and branding, but to continue collaborating with ONC as a potential customer for help desk services. Other government entities may have the same needs. HL7 could consider charging for these services. IP Policy Issues IP policy issues were presented and discussed at some length but without significant resolution. See separate notes on the Intellectual Property Licensing Taskforce below for background and next steps. Healthcare Provider membership The HL7 rules currently require that a healthcare provider member be treating or seeing patients. The particularly favourable rate offered to healthcare providers is intended to encourage them to participate in HL7 activities—particularly requirements definition—but it has not been widely taken up. It was considered that if more clinicians who are primarily engaged in senior health informatics roles within organisations were to take up membership as a healthcare provider, it might encourage both their participation and ultimately more organisational memberships. The HL7 Board accepted the committee’s recommendation to extend the eligibility for ‘healthcare provider membership’ to include anybody that ‘is currently seeing patients’ or ‘has 10 years of patient care experience and is currently working for a healthcare organisation in an administrative role’. Timelines It was agreed that the Membership Committee would progress its work in accordance with the following timelines— • ongoing refinement of benefit definitions and recommendations based on additional research data; • developing recommendations on benefits that require only focused review for the July Board meeting; • reviewing the communication aspects of the proposed benefits to ensure consistency for the July board meeting; • conducting overall review and finalisation of recommended benefit packages and recommending a dues structure for initial implementation for the July Board meeting (ensuring that there is sufficient differentiation across membership levels); and • defining a strategy for growing membership for Board consideration at the September 2013 plenary meeting. 2.5.2.2 INTELLECTUAL PROPERTY LICENSING TASKFORCE The various HL7 International license agreements contain restrictions on the ability of recipients of HL7 documents to distribute them further, or to create and distribute ‘derivative works’ depending on whether they are members, non-members or affiliate organisations. Under the current HL7 International IP policies the following applies: • Organisational members of HL7 International and its affiliates are permitted to share excerpts of HL7 IP with their customers, but individual members and non-members are not permitted to do this. 33 Final Report HL7 Meeting—Atlanta, USA (May 2013) • Organisational members of HL7 International and its affiliates are permitted to develop ‘derivative works’ (in particular implementation guides—IGs) but may only distribute them to their customers. In reality this is difficult to enforce. • No one (whether they are an HL7 member or not) is permitted to republish original HL7 specifications on public websites. Affiliates may distribute copies of HL7 specifications to members within their territories, but always subject to the HL7 International IP policy. • No one is authorised to publish derivative works (including IGs) containing HL7 IP on a public website, however a number of government agencies do this (some being members and some not) and it is difficult to enforce—particularly against a non-member that did not download the original work. • There is reluctance on the part of governments to adopt HL7 standards, because their eHealth authorities may be precluded from widely distributing implementation guides that they develop. • The HL7 vision ‘To create the best and most widely used standards in healthcare’ is difficult to achieve. • Individual organisations can obtain special permission for reasonable use of HL7 IP by getting approval via the HL7 International Executive Committee, but this is not widely recognised or necessarily acceptable to public authorities. HL7 Australia is one of the few organisations to get such an agreement in respect of HL7 Implementation Guides as Australian Standards and lower consensus documents prepared by Standards Australia and published through SAI Global. The need for the HL7 Board to decide whether current policies and licensing terms should continue or be revised was discussed, and it was agreed that further more detailed work is required to inform the activities of the Membership Committee and any further decisions of the HL7 Board. Until a decision is taken the HL7 administration will continue to adhere to the long established policy of asking those who are publishing to become members or cease their unauthorised use. To inform further work of the Membership Committee and decisions of the HL7 Board, it was resolved that and IP Licensing Taskforce would be established (which may include representatives who are not on the Membership Committee) under the Membership Committee to review IP policies and provide recommendations to the Board for discussion at the Board retreat. It was noted that Richard Dixon Hughes, Keith Boone and Philip Scott would be among those willing to serve on the taskforce, which would be convened by the Membership Committee. 2.5.3 CEO REPORT Topics addressed during presentations and reports by the CEO (Chuck Jaffe) included: MEMBERSHIP COMMITTEE The Membership Committee is a board-appointed standing entity responsible for developing recommendations about membership benefits and a new dues structure. A large part of the various CEO reports during the WGM summarised key elements of the activities and recommendations of the Membership Committee, including— • the composition and role of the Membership Committee and its evolution from the consultation and other work carried out by the previous Membership Taskforce; • the substantial polling and market research that had been undertaken of both HL7 members and nonmembers since March 2013, including at HIMSS and by telephone and internet surveys; 34 Final Report HL7 Meeting—Atlanta, USA (May 2013) • the four enduring principles underpinning this work—consensus, collaboration, communication and trust—underpinned with a need for good humour; • the identification of 14 areas of potential member benefit and the work being done to assign these to different levels and categories of members and non-members, and the basis for a fair and equitable dues structure; • the implementation impacts, including any further changes to IP policies and investments required to deliver new benefits; • the areas for decision by the HL7 Board—who gets what and for how much, IP policies, investments in new capability, and implementation timelines; and • a commitment to informative monthly updates on progress toward the introduction of new membership benefits, organisation and directions coming into effect from October 2013 (and to be presented at the September WGM and plenary). A considerable portion of the face-to-face Board time at this WGM was devoted to discussing recommendations of the Membership Committee [as reported above]. FREELY ACCESSIBLE STANDARDS Individual HL7 standards became available for free download from 1 April 2013 from the HL7 website under an updated license agreement. The initial implementation required a questionnaire to be completed for each document downloaded and this was of concern to those promoting the universal adoption and use of HL7 standards. This restrictive approach had been accepted as a problem, and the site was being redesigned to enable multiple documents to be downloaded based on a single response to the questionnaire and license terms. Requests from the media continue to be received seeking information on HL7’s decision to make its standards and other selected IP available free of charge. The various license agreements contain restrictions on the ability of recipients of HL7 documents to distribute them further, and to create and distribute ‘derivative works’ depending on whether they are members, non-members or affiliate organisations. The need for a Board decision on whether current policies and licensing terms should continue or be revised was highlighted and discussed [resulting in the formation of an IP taskforce as reported elsewhere]. CHANGES IN HL7 EXECUTIVE SUPPORT PERSONNEL The following recent additions to the HL7 International Resource Team were noted: Dr Sharon Chaplock—Director of Education: • Since commencing in January, Sharon has been working on a significant expansion of HL7 International’s educational offerings and activities. This commenced with a popular new series of webinars on topical issues, with new approaches, new coursework and new technology now being planned. • The new education portal is due for initial release in June 2013 with a catalogue of offerings and progressive enhancement over the coming year. 35 Final Report HL7 Meeting—Atlanta, USA (May 2013) • Online testing for HL7 professional certification is expected to become available through the education portal in Q3/2013, with plans for it to be available internationally through approved examination centres. • Various professional and technical organisations are considering reuse of the HL7 eLearning program, either as a stand-alone entity or through integration into more broadly based training programs. • HL7 Australia has noted that this expansion in HL7 International training and certification services may impact on some services traditionally offered by affiliates and some third party HL7 training organisations operating in their territories. Nevertheless, there are more potential synergies than competitive disadvantages, even in countries (such as Australia) which do not have the natural barrier of requiring training in a language other than English. Ticia Gerber—Director of Global Partnerships and Policy: • Ticia commenced on 1 May after having held various policy and partnership development roles over many years, including recent work with the WHO (eHealth strategy), the Rockefeller Foundation and the eHealth Initiative. She will operate out of donated office space in Washington DC. • She was present at the WGM, and gave presentations to the Board and Affiliate Chairs introducing herself, her role and aspirations (summarised below as a separate topic). Carol Carmick—Director of Marketing: • Carol is due to join HL7 International in June and comes with an over 20 years in marketing of HIT software, business solutions and professional societies (including some work for HIMSS). • Her responsibilities will include the development of marketing and communications campaigns, new global marketing initiatives, data gathering and analysis, branding and re-branding activities, and working with WGs and committees (particularly on the identification, packaging and marketing of product families and product lines). They join other longer-established members of the HL7 resource team in the areas of: • Operations—Chuck Jaffe (CEO), Mark McDougall (Executive Director) and Karen Van Hentenryck (Associate Executive Director) • Strategic Planning—Virginia Riehl (contract consultant) • Membership Services—Diana Stephens • Communications—Andrea Ribick HL7 BALLOTING OF IHE PROFILES Implementation of the agreement between HL7 international and IHE international to ballot relevant IHE profiles through HL7 is proceeding. Following the recent IHE technical meeting, significant challenges have been recognised in relation to validating the process, defining objectives and selecting relevant profiles. To resolve these issues there will be an IHE balloting pilot conducted by HL7 for which the initial profiles/IGs have yet to be determined. Opportunities for the pilot to support ONC profiles for RFD (Retrieve Form for Data Capture) and data segmentation for privacy are being considered. The CEO has recommended that an HL7 taskforce be established to liaise with IHE counterparts in order to develop process, select pilot artefacts and establish deliverables. 36 Final Report HL7 Meeting—Atlanta, USA (May 2013) OTHER COLLABORATION AND OUTREACH A strong collaborative and outreach program continues to be pursued with the following being particularly noted: • Current and/or planned HL7 Europe involvement in European Commission projects and activities. • Proposed activities of HL7 Asia, which is now operating with funding from respective ministries of health in China, Japan, Korea, Taiwan, Hong Kong and Singapore. • Big data—interest in the potential applications of big data in health continues to explode, particularly with the JAMIA special issue on big data, and the emergence of the Apixio platform and the Triad DataSpace technology for secure storage and analysis of big data sets. • The CEO recommended that the TSC investigate the possibility of a work item proposal and leadership of a project on potential HL7 engagement on big data. • IHIC 2013—support is encouraged for this event being hosted by HL7 Australia in Sydney on 28– 29 October 2013. • Paediatrics engagement—with support of the Vanderbilt Informatics group (Chris Lehmann) and the American Academy of Pediatrics (AAP), the following are now progressing— • - reactivation of the HL7 Child Health WG; - CDA pediatrics template development; - Electronics Pediatric Survey project; and - Portable Health Record, using the PHR-FM and modelled after an existing paper profile. CEO news brief—the first issue was emailed to the membership in May. Critique and suggestions are sought. • CIMI support—a financial proposal for HL7 support of CIMI development and meetings was delivered to the CIMI International Executive Council. Response was awaited. 2.5.4 2.5.4.1 OTHER NON-PROJECT WORKING GROUP DISCUSSIONS AND PRESENTATIONS TREASURER’S REPORT Calvin Beebe presented the Treasurer's report, which addressed both the final 2012 financial results and audit report, and the preliminary indicators of performance for the 2013 year. Key figures from these two sets of results are presented in the following consolidated table. 37 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2012 Actual 2013 YTD 2013 Y/E Budget Revenue $5.364M (17.5% or $700K favourable) $1.868M (slightly favourable) $4.383M Expenses $4.564M (6.3% or $305K favourable) $1.261M (slightly favourable) $5.042M Surplus/(Deficit) $800k ($1.1M favourable) Slightly favourable to budget ($659k) deficit Reserves ($) $5.377M $4.803M $4.762M Reserves (months) 14.14 months 13.10 months 11.4 months Most of the much better than expected financial performance in 2012 can be attributed to organisational membership revenues coming in $618K (or 25%) over budget. A strong performance was also recorded for distance learning, off-site workshops and certification testing—revenues from these sources were $150K (or 50%) favourable to budget. Delays in filling the Director of Education position due to unexpected illness of the candidate first selected caused a shortfall in staff costs for 2012, however this saving came at the cost of significant delays in progressing the education strategic plan and the benefits to flow from it. Closely tracking any financial implications of the recent IP policy change is an important priority for the Finance Committee, which monitors the net retention rates of organisational members each month. Figures for the first four months are inconclusive but suggest the possibility of a slight down-trend in numbers and revenue. If the Financial Committee determines that a significant adverse trend is occurring, it will alert the HL7 Board. 2.5.4.2 HL7 TERMINOLOGY AUTHORITY The Board approved a TSC recommendation to form the HL7 Terminology Authority (HTA) as a gatekeeper to ensure that content from HL7 is submitted to IHTSDO in a controlled manner. It was noted that formation of the Authority— • is required under the agreement between IHTSDO and HL7 International; • enables HL7 International to moderate and register SNOMED CT concepts under IHTSDO rules; • helps HL7 International to provide timely, high quality terminology products and services to meet its business needs; and • provides a single point of communication with external terminology SDOs with which HL7 has formal relationships. The HTA operates as follows: • If terminology content already exists, then HTA would not take any action. • If new content is required for the universal realm, the request would come through the HTA. 38 Final Report HL7 Meeting—Atlanta, USA (May 2013) • If new content is realm specific, the request for new content would go through the relevant IHTSDO endorsed national release centre (NLM for the US and NEHTA for Australia). The activities and operations of the HTA will be tightly coupled with the HL7 Vocabulary WG (of which Heather Grain of Australia is a Co-Chair). The NTA will have five members experienced in the maintenance of clinical terminology who will be nominated by the CTO and appointed by resolution of the HL7 Board. At least two of the members will be drawn from among the affiliates. A call for nominations will be issued. 2.5.4.3 MAY 2015 WGM IN EUROPE Based on further investigation and reports of financial projections showing a considerable loss, the Executive Committee had recommended against having the May 2015 working group meeting in Paris as previously planned (their preference was Montreal). After some discussion and noting that several recent meetings planned outside North America had been cancelled, the HL7 Board rejected the Executive Committee’s recommendation and resolved to continue with plans to hold a European working group meeting to be hosted in France. The WGM is to be organised on behalf of HL7 Europe as a whole and not just HL7 France, although HL7 France (Interop Santé) would continue to have the lead role. Other European affiliates (notably the UK, the Netherlands, Switzerland and Germany) pledged their support. The HL7 Board’s approval is subject to a European organising committee being set up that presents the HL7 Board with a set of measurable and strategic objectives. Phillip Scott (UK) volunteered to work with Nicholas Canu (France) to ensure that this occurred. After some consideration of relevant European holidays and potential competing events, it was confirmed that the meeting would continue to be planned for the proposed venue near Paris in the week of 10 May 2015. 2.5.4.4 DIRECTOR OF GLOBAL PARTNERSHIPS AND POLICY The recently appointed Director of Global Partnerships and Policy—Ticia Gerber—was introduced by the CEO. It was noted that her background included— • nearly two decades working with legislators, regulators, international agencies, donors and civil society to achieve public policy change, and identify the funding and cooperation needed for better health; • being a key figure in securing bipartisan congressional support for eHealth legislation within the United States while serving as Vice President of Public Policy at the eHealth Initiative; • leading a WHO project, facilitating the development of policy implementation knowledge and resources for health information systems; and • advising the Rockefeller Foundation on eHealth and health system transformation issues, working to draft and secure an international eHealth call to action, and compose the first comprehensive publication on eHealth in the developing world. She spoke to the key responsibilities of her role at HL7, which has two interrelated aspects—a global partnerships perspective and a policy development perspective. 39 Final Report HL7 Meeting—Atlanta, USA (May 2013) The global partnerships aspect is involved with working with organisations to attract funding and investment in and through HL7, in order to— • raise awareness of HL7, increasing its role and funding portfolio at both globally and at national level; • secure actionable, funded projects for HL7 through strategic outreach to potential donors and partners, achieving funding of up to US$1 million in new partnership and policy initiatives within 12 to 18 months; • gather relevant market intelligence and engage with up to 100 potential donors and partners in the fields of global health, international development, ICT and to government, academia and industry within a few months; and • strengthen existing HL7 relationships with the US federal government and globally relevant organisations with an eye towards increased grants and funding. The policy development aspect is involved with— • increasing HL7’s policy capacity and member resources by building an active advocacy community among HL7 members; • working to secure policy environments that enable the achievement of HL7’s vision, mission and priorities, and engaging policy powerbrokers to boost HL7 policy presence and funding; • determining HL7’s unique policy contribution and member needs; and • developing strategies to leverage HL7 education and communication channels to inform members of policy issues, and raise the eHealth policy profile of HL7 and its members. Achievement of these outcomes will involve collaborating closely with the HL7 Resource, Communications, Education and Marketing teams, as well as groups such as the Policy Advisory Committee. When asked about what would differentiate her approaches at HL7 from those of her previous clients, Ms Gerber indicated that she was particularly encouraged by the recent opportunities offered by HL7’s recent decision to make its standards and other selected Intellectual property freely available. Those who have had previous dealings with Ms Gerber were all enthusiastic about the potential contribution that she could make to the HL7 community. 2.5.4.5 OTHER HL7 BOARD BUSINESS CEO and CTO performance goals for 2013 as recommended by the CEO/CTO evaluation taskforce were discussed and approved. Providing more measurable goals has been a key focus for the committee on this occasion, responding to feedback from HL7 Board members. The Chief Technical Officer (CTO)—John Quinn—also reported briefly, mainly covering matter addressed in his other reports to the WGM. The following key points were noted. • SKMT (Standards Knowledge Management Tool)—use of this tool is being promoted within HL7 as a means of harmonising HL7 terminology, and incorporating HL7 terms into the shared health informatics glossary that is being maintained by ISO/TC 215 and supported by the Joint Initiative Council (JIC). • Because all HL7 terms are assigned to a single HL7 document within SKMT, all HL7 contributors have the capacity to wreak havoc by making uncontrolled changes to terms and definitions entered by other HL7 contributors. HL7 contributors to SKMT were therefore asked to work with the Vocab WG, the CTO and other contributors in adding or changing HL7 material within SKMT, and only change definitions for which they had some clear level of responsibility within HL7. 40 Final Report HL7 Meeting—Atlanta, USA (May 2013) • RIM in OWL—the HL7v3 RIM has been successfully generated in OWL and checked for validity. This should allow it to be ported to other OWL environments and used by inference engines. The resulting XML representation (approximately 300 pages in a single file) has been judged to be valid, although far from elegant. • The second part of project will be finished in August. This should allow the RIM to be imported into off the shelf tools. • There are plans to release the utilities to create the OWL XML file before the next meeting. In the meantime the current XML file (one rev behind the current RIM) will be posted on the HL7 website for download and experimentation. The CTO noted that it is quick and easy to download and install Protégé from the internet and then import the XML file. • Those with an interest in tooling are encouraged to try to for themselves and investigate how this resource can be used: - Tooling next steps—the Tooling WG and CTO will embark on a tooling tactical plan prioritised by the decisions of the membership, policy committees and the Board as soon as those decisions are made. - Involvement of HL7 in ISO/TC 215—John Quinn as CTO also participates in meetings of some other SDOs representing HL7 International, asking questions and making suggestions on its behalf. He noted that there were at least 16 other active HL7 members present at the ISO/TC 215 meeting in Mexico City from 20 to 26 April 2012. • The CTO indicated that he would like to better coordinate the activities and improve the effectiveness of HL7 at ISO and other meetings. He requested any others that take part in meetings of other SDOs to make them known to him, so that he can work with them to achieve this goal. In addition to the above matters, the following regular Board reports for information and possible action were noted: - membership reports (including voting members, benefactors, 2013 statistics and detailed history of affiliate membership information); - education WG reports on status of the Education Strategic Plan and statistics on Meaningful Use (MU) webinars; - IHTSDO liaison officer’s report (Russell Hamm); - Regenstrief/LOINC liaison officer’s report (Ted Klein); - report of HL7 liaison to ISO/TC 215 on meeting in Mexico City (Ted Klein); - V3 Project Report—first trimester 2013 (Woody Beeler); and - various other reports from committee and WG liaisons for: Electronic Services WG, International Mentoring Committee, Organisational Relations Committee, Process Improvement Committee, Project Services WG Publishing WG, and Tooling WG. 41 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.5.5 ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by HL7 Board: Issue: Changes to the HL7 membership model, membership benefits, education and certification services, and IP licensing policies have potential consequences for the HL7 affiliates, including HL7 Australia. Although generally positive for worldwide adoption and acceptance of HL7 standards, these changes may impact the perceived benefit of being an HL7 Australia member, and other HL7 Australia offerings and its contractual relationships. HL7 Australia Changes to HL7 International membership model, services and licensing policies Action: HL7 Australia will continue monitoring, analysing and responding to the emerging changes in the HL7 International membership model, services and IP licensing policies. HL7 Board: IP Licensing Taskforce Issue: HL7 international has formed an IP Licensing Taskforce to provide advice through the Membership Committee on IP licensing issues arising from free licensing of HL7 standards. The IP Licensing Taskforce will also provide advice on potential interactions with the new membership model, including possible difficulties in relation to the different treatments accorded to derivative works under various contractual arrangements. Richard Dixon Hughes has been appointed to the taskforce based on his legal qualifications, his current role as Chairman of HL7 Australia, and his knowledge of agreements between Standards Australia and HL7 Australia in relation to the use of HL7 International IP in Australian Standards and lower consensus publications. Chair of HL7 Australia Action: Richard Dixon Hughes is to participate actively as an appointed member of the HL7 International IP Licensing Taskforce, with a view to ensuring consistency between the various types of IP licenses, the Affiliate agreement and other contractual arrangements for use of HL7 International’s intellectual property. HL7 Terminology Authority (HTA) Issue: The HL7 Board has accepted a recommendation to form an HL7 Terminology Authority (HTA) under the agreement between IHTSDO and HL7 International, which will act as a gatekeeper to ensure that content from HL7 is submitted to IHTSDO in a controlled manner. Of the five members of the Authority, two must come from within the HL7 affiliates, be appropriately qualified and experienced, and be prepared to participate regularly in required teleconference calls. A call for nominations is to be issued. Appropriate Australian candidates should be given the opportunity to nominate. HL7 Australia Standards Australia Action: The HL7 call for nominations of suitably qualified persons to serve on the HTA will be circulated to IT-014-02, IT-014-06 and NEHTA (Terminology Services). 42 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.6 CHILD HEALTH 2.6.1 PROGRESS AT THIS MEETING The Child Health workgroup reconvened at the Atlanta meeting and confirmed three new Co-Chairs. Formal voting for Co-Chairs will be conducted at the September meeting. At the meeting this group identified a number of work items which would likely form its three year work program— • reaffirming the EHR functional profile release 1 for child health, and following up with the development of new functional requirements for inclusion in release 2; • neonatal functional profile (a continuation of the EHR functional profile activities); • portable health record; • EPSDT (early and periodic screening, diagnostic and treatment) milestone template; • paediatric nutrition and diet; • anthropometrics template; and • developmental surveys electronic question and response. Portable health record—this is a record for parents to carry in case of an emergency. The content includes problems, medications, allergies, healthcare provider details and special instructions on treatment or procedures such as vascular access (which is important for children with chronic or potentially lifethreatening conditions). It uses CDA templates. EPSDT milestone template—this is also a CDA template based project. It includes comprehensive physical examination of the eye and vision, hearing, the teeth and mouth, the head and neck, the heart, and milestones such as response to sound, rolling, behavioural observations, immunisation and a nutrition section. Assessments are done at birth, 1 month, 2 months, 4 months, 6 months, 9 months, 12 months, 15 months, 18 months, and 18–21 years old. The content forms part of a child’s longitudinal record, and is exchangeable with a personal health record (PHR) and EHR. Anthropometrics template—this covers the height and length, weight, head circumference, and percentiles based on growth charts. Nutrition information—this includes diet and feeding history, food allergies, dental history, and developmental or medical issues that impact on nutritional status. 2.6.2 ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by Child Health: Issue: These potential projects are relevant to Australia’s PCEHR Child eHealth Record. IT-014-06-06 EPSDT Milestone and Anthropometrics Template Action: The Australia e-Health community, specifically IT-014-06-06, IT-014-13 and NEHTA, should observe these closely, and provide input to the development of these templates. IT-014-13 NEHTA 43 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.7 2.7.1 CLINICAL DECISION SUPPORT PROGRESS AT THIS MEETING The WG is working hard to get back to ‘green’ and achieving a gold star to indicate a healthy working group. Unpublished ballots include VMR CDS V2 IG R1 (DSTU ballot this cycle) and CDS Knowledge Artefacts implementations R1 (needs DSTU pub request). A lot of time was dedicated in this WGM to reconciliation of the Virtual Medical Record (VMR) ballot comments. This ballot was affirmative. A new project for primary sponsorship by the CDS work group was presented—Expression Language Functional Requirements. The scope statement is reported to be: ‘To develop a set of conceptual requirements based on existing HL7 work products for expression languages used to complete results. It is not creating new functional requirements for which we do not have existing examples of in existing expression languages. Nor is it to determine what requirements must be met by an expression language. Rather, it is a catalogue of requirements that have already been applied.’ There was a suggestion from the US VA that this work wouldn’t be particularly helpful or likely to be taken up by Meaningful Use. A motion was passed to establish a new project with a scope joint among SD, CDS and CQ to define the requirements for expressions based on extant HL7 expression languages and work for the January 2014 ballot. Outlook for new projects for this work group were also discussed as— (1) CDS Guidance Service Implementation Guide—new PSS, DSTU (normative track with conformance criteria); (2) Decision Support Service revision with a new project scope statement submission and ballot new release (go straight to normative if minor changes, go DSTU then normative); (3) VMR Domain Analysis Model revision with a new PSS for new release; (4) VMR XML Implementation Guide revision with a new project scope statement for the new release; and (5) CDS knowledge artefact implementation guide (go through DSTU again as a next release with a new project scope statement). A motion was passed to revise the four last project items. The agenda for the Cambridge meeting was also set. The WG confirmed that they would keep the same meeting days and set the provisional meeting dates of Wednesday, 25 September 2013 to Thursday, 26 September 2013. The planned agenda includes review work on Infobutton, VMR, CDS Knowledge Artifact IG. A joint meeting is planned (hosted by Structured Documents) to discuss VMR/QDM harmonization and the proposed new Expression Functional Model project. 2.7.2 CURRENT PROJECTS The following projects are reported on in this section: • Project #931: Implementation Guide: Clinical Decision Support Knowledge Artefact, Release 1 • Project #875: Context-Aware Knowledge Retrieval, Knowledge Request, Release 2 • Project #876: URL Based Implementations of the Context-Aware Information Retrieval (Infobutton) Domain, Release 4 • Project #184: Virtual Medical Record (vMR) for Clinical Decision Support 44 Final Report HL7 Meeting—Atlanta, USA (May 2013) • Project #259: Order Set Publication Standard • Project #863: Cross Paradigm Interoperability Implementation Guide for Immunisations • Project #924: Healthcare Coordination Services 2.7.2.1 PROJECT #931: HL7 IMPLEMENTATION GUIDE: CLINICAL DECISION SUPPORT KNOWLEDGE ARTEFACT IMPLEMENTATIONS, RELEASE 1 PROJECT OBJECTIVE The project aims to provide guidance that facilitates the ability of content providers to create CDS artefacts in a manner easily consumed by systems that provide CDS, and through CDS Knowledge Sharing support widespread use of artefacts to improve care. There are a number of standards that currently exist to address this need but to date there has been no coordinated effort to bring these standards together. The project addresses the need to identify how to use each of these standards and a process for structuring the implementation guidance of those artefact types that are currently not supported through existing standards. This work effort will encompass the creation of a Domain Analysis Model (DAM) and associated IGs for CDS Knowledge Sharing, which is informed in part by the requirements developed as part of the Standards and Interoperability Framework's Health eDecisions initiative. To the extent possible, this project will leverage and harmonise existing standards such as Arden Syntax, Order Sets, vMR and GELLO. The project will include a gap analysis, recommended changes to existing standards, and recommendations for pre-adoption of modifications and specifications that close the identified gaps. Additionally external (nonHL7) standards and specifications may inform the work to close those gaps. PROJECT ACTIVITY/ISSUES AT THIS MEETING Health eDecisions status update Various pilots are currently in testing for use case one—authoring ordersets in CDS. So far the pilots look successful. Use case two—CDS guidance service—is under ballot planning for September. Currently a decision is needed between the use of DSS, SOAP, REST, Consolidated CDA or VMR. The goal is to ballot this second use case in September. 2.7.2.2 PROJECT #875: CONTEXT-AWARE KNOWLEDGE RETRIEVAL, KNOWLEDGE REQUEST, RELEASE 2 PROJECT OBJECTIVE This project aims to revise the current standard, the supplement to the current standard and the IG. It will be an enhancement of the earlier release of the normative standard Context-Aware Knowledge Retrieval— Knowledge Request. Additional information can be located at the HL7 website (http://www.hl7.org/Special/committees/dss/projects.cfm?action=edit&ProjectNumber=875). The Context-Aware Knowledge Retrieval (Infobutton)—Knowledge Request specification provides a standardised knowledge request mechanism to act on knowledge resources such as Health Record systems, so as to aid at the point of care by delivering both relevant clinical information and patient centred education materials as relevant to the clinical context. The Release 2 project addresses use cases requested by implementers. These include the ability to specify additional patient clinical attributes and geographical locations of interest, the specification of a healthcare 45 Final Report HL7 Meeting—Atlanta, USA (May 2013) payer as the performer or information recipient of an Infobutton request, and clarification of the description of the modelling. PROJECT ACTIVITY/ISSUES AT THIS MEETING This DSTU was submitted for publishing following the January 2013 WGM. No further update was available at this meeting. 2.7.2.3 PROJECT # 876: URL BASED IMPLEMENTATIONS OF THE CONTEXTAWARE INFORMATION RETRIEVAL (INFOBUTTON) DOMAIN, RELEASE 4 PROJECT OBJECTIVE The aim of this project is to revise the current standard to develop an IG specifying URL based implementations of the Context-Aware Knowledge Retrieval (Infobutton) Standard. It is intended as an HL7 Informative Document. It will support implementations that use URLs as communication protocols with the ultimate goal of enabling a transition from URL based implementations to a services oriented approach. Release 4 of this IG reflects changes made to its normative parent specification, Context-Aware Knowledge Retrieval, Knowledge Request Standard. It also includes clarifications for the use of coded attributes and provides a quick reference of code systems used in the IG. Being URL based and employing HTTP, applications built around this implementation guide require less understanding of HL7 V3 and could be simpler for vendors than the base Infobutton standards. Further information can be located at the HL7 website (http://www.hl7.org/Special/committees/dss/projects.cfm?action=edit&ProjectNumber=876). PROJECT ACTIVITY/ISSUES AT THIS MEETING Infobutton project was discussed in a joint meeting with the SOA WG. IG Ballot Reconciliation was completed. Guilherme Del Fiol provided an update regarding the various artefacts pertaining to the Infobutton standard. This particular first normative ballot was approved (affirmative = 58, negative = 0, abstention = 81). Ballot comments were reviewed and considered, dispositions were determined, and reconciliation was completed. 2.7.2.4 PROJECT #184: VIRTUAL MEDICAL RECORD (VMR) FOR CLINICAL DECISION SUPPORT PROJECT OBJECTIVE The objective of this project is to create HL7 VMR data models capable of supporting scalable, interoperable CDS. The VMR uses only the CDS essential EHR abstractly specified data. It is thus independent of implementation technologies including HL7 V2, V3 messaging and V3 CDA. It encourages CDS at the point of care by reducing costs and response turnaround time. It eliminates the need for EHR vendors to maintain proprietary CDS structures and messages. It is of great value to emerging clinically focussed applications which address patient outcomes and community needs. VMR is being further developed with more IGs and template specs for standard data models including CCD, Care Plan of Patient Care and priority tasks of CDS (e.g. Drug to Drug interactions, vaccine advice, Family History Risk Analysis and Genomics). Transformations are being developed between standard models which are moving towards greater alignment with HL7 work. 46 Final Report HL7 Meeting—Atlanta, USA (May 2013) The VMR has become a foundation source of modelling input for the ONC S&I Framework’s IG for Knowledge Artefacts, which will be part of MU 3 (see project description above). Further information can be located at the HL7 website (http://www.hl7.org/Special/committees/dss/projects.cfm?action=edit&ProjectNumber=184). PROJECT ACTIVITY/ISSUES AT THIS MEETING VMR DAM Release 2 Ballot Reconciliation has been conducted. The ballot passed by the numbers (first informative ballot) with 36 voting affirmative, 5 voting negative and 93 abstaining. Ballot comments were reviewed and considered, dispositions determined, and reconciliation was completed. Main changes included the removal of the SOAP specification, a revised and clarified RESTful and knowledge response specification, a redesigned ATOM category specification, and an added JSON knowledge response. Next steps will likely include the synchronisation and consolidation of the two implementation guides into one specification. A number of diet related comments were reconciled as provided by the International Dietetics group. These primarily related to naming changes to ensure consistent terms with the Diet Order DAM. These included enteral feeding, feeding volume and caloric density. Lantana raised the issue that a VMR problem may include items that would not be in a patient’s problem list, such as an encounter diagnosis from a past encounter or an inactive problem. There appeared to be some confusion about definition of a problem list, and whether this would include inactive problems and diagnoses (not just current problems). Discussion was dedicated to whether a template would need to be included, and the clinical quality and harmonisation of what could be done in this space. The note was also made that the model lacks classes for communications. This will be evaluated by Health eDecisions pilot activities and incorporate a refined model as appropriate following review in an HL7 work group call. Andrew McIntyre (Australia) presented the VMR V2 Implementation Guide. Ballot comments were reconciled and the ballot was passed. The target for this project is that HL7 publishes HL7 vMR DAM Release 2 as an Informative Specification with the target date being the September 2013 WGM. There was some general discussion about how FHIR may make vMR redundant. The suggestion was made that if this was the case within the community then it may be appropriate to consider. VMR V2 Implementation Guide Andrew McIntyre presented the topic of this project. VMR V2 is a batch of messages. The agenda was to discuss and resolve ballot comments submitted by OO members who unfortunately were not present at the meeting. However the members in attendance were able to continue the resolution process. It is expected that this project will be the basis for an Australian implementation guide. Problematically, there is a desire to use more than 10% of the HL7 International content in the Standards Australia Implementation Guide. This would be a breach of the terms of the Standards Australia agreement with HL7 Australia and HL7 International. Daniel Henzi (SA) raised the matter with Richard Dixon Hughes (HL7 Australia Chair) and Nat Wong (HL7 Australia Secretary). Richard and Nat noted that while an exception might be possible, neither felt that HL7 International would see a reasonable case for such an exception. Nat noted that it would be much simpler if the Australian IG for this project followed the same rules as other Australian IGs and referenced the HL7 International Standards work appropriately, while also acknowledging that this would mean that the Australian IG could not be read without heavy reliance on the HL7 International VMR project. 47 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.7.2.5 PROJECT #259: ORDER SET PUBLICATION STANDARD PROJECT OBJECTIVE This project has developed a special instance of a structured document standard and associated IGs for the publishing and sharing of interoperable order sets for clinical system vendors to deploy for CDS at point of care. It is really a knowledge document, not an instantiated order set. Further information can be located at the HL7 website (http://www.hl7.org/Special/committees/dss/projects.cfm?action=edit&ProjectNumber=259). Order Sets are one of the three knowledge artefact groupings in the ONC S&I Framework’s IG for Knowledge Artefacts that will be part of MU 3 (see project description above). The HL7 Order Set Standard is closely aligned with that part of the ONC S&I IG. PROJECT ACTIVITY/ISSUES AT THIS MEETING No update was available at this meeting. 2.7.2.6 PROJECT #863: CROSS PARADIGM INTEROPERABILITY IMPLEMENTATION GUIDE FOR IMMUNISATIONS PROJECT OBJECTIVES The CDS WG and the PHER WG are co-sponsors with SOA of this SAIF framework project. This was worked on in common with the CDC immunisation evaluation and forecasting logic specification project, and immunisation use cases have been incorporated. Semantics of Business Vocabulary and Business Rules (SBVR) is used and RuleSpeak is under consideration. The project is factoring out common material from a large and perhaps challenging number of multiple platforms and models to develop a common information and common behavioural model. Arden Syntax and VMR have previously been recommended by CDS for consideration in this work but currently not adopted. PROJECT ACTIVITY/ISSUES AT THIS MEETING No update was available at this meeting. 2.7.2.7 PROJECT #924: HEALTHCARE COORDINATION SERVICES PROJECT OBJECTIVE This is a SOA project with Patient Care (PC) and CDA WGs as co-sponsors. It aims to create a standard and other specifications for an HL7 SOA Care Coordination Service to support collaboration amongst a multidisciplinary care team, consisting of members from either the same or different organizations (e.g. primary care clinic, home care, allied health professionals, hospital or skilled nursing facility). It is intended for real time deployment, meaning that changes made by one participant are propagated to all. All stakeholders and collaborators in a patient’s care can make updates to the care plan, update health concerns and add supporting detail relevant to the care record. The Care Coordination Service (CCS) interface will expose a consolidated view to all participants. It is a healthcare socio-technical standard for enabling live collaboration among care team participants. 48 Final Report HL7 Meeting—Atlanta, USA (May 2013) PROJECT ACTIVITY/ISSUES AT THIS MEETING This is a SOA project with Patient Care (PC) and CDA WGs as co-sponsors. No input from the CDS WG was given at this meeting. 2.7.3 ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by Clinical Decision Support: Issue: Infobutton standards address relatively simple interactions with EHR based applications, and address both patient and provider knowledge needs. US implementation is required for Meaningful Use 2. These needs are universal, and the standards offer benefits to Australian healthcare providers, consumers and vendors. IT-014-13 Infobutton Action: Ongoing development of Infobutton standards in the Australian context to be informed by international developments. Clinical Decision Support: Virtual Medical Record (vMR) for Clinical Decision Support Clinical Decision Support: Virtual Medical Record (vMR) for Clinical Decision Support Clinical Decision Support: VMR v2 Implementation Guide Issue: Standards Australia’s CDS project HL7 V2 Virtual Medical Record Profile is feeding into HL7 International’s CDS and V2 work and vice versa. IT-014-13 Action: International development in the vMR work and IGs to continue to inform HL7 V2 Virtual Medical Record Profile project in Australia. Issue: Incorporation of nutrition and diet specifications is included in the vMR from the Diet Order DAM. Such requirements are important for decision support and patient safety for dieticians, speech pathologists, physicians, nurses and their patients in Australia. Action: It will be important for take up in the Australian context that these specifications for decision support are included and modelled on international Diet Order specifications. Other allied health requirements should also be explored for decision support. Issue: A relaxation of the amount of content of HL7 International Intellectual Property included in Standards Australia Implementation Guides may be required for this project, or in the absence of such a relaxation a review of the manner in which this Australian Implementation Guide will be structured. IT-014-13 Allied Health Professions Australia (AHPA) Standards Australia HL7 Australia Action: Review proportion of original HL7 content in CDS implementation guides. 49 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.8 CLINICAL STATEMENT 2.8.1 2.8.1.1 PROGRESS AT THIS MEETING CLINICAL STATEMENT (CS) MODEL The CS model completed a normative ballot in May 2013. There were about four negative ballot comments to be resolved. One of the negative comments required a couple of structural codes to be added to the mood code of the Supply Act class. The group voted to accept the ballot comments and recommended the addition of the structural codes as required. This was considered to be a significant change and vocabulary harmonisation. Resubmission of the CS ballot after vocabulary harmonization for re-ballot in September is required. A motion was passed to accept the reconciliation recommendations, withdraw the CS model from normative publication and prepare for the September 2013 ballot. 2.8.1.2 TOOLING LIAISON UPDATE The CS workgroup is regularly required to perform model harmonisation between the CS model and models produced by other domains or workgroups that impact on the structure and semantics of the CS model. This is currently a manual process, which is not only extremely labour intensive but also error prone. There is a requirement for an automated model-to-model transform checker or validator. More generalised use cases will also benefit from the use of such automated model validation: To check that a particular RMIM is a proper constraint of its parent DMIM, including conformance to classes, attributes, relationships, datatypes, cardinalities, conformance and vocabulary/terminology. However such a tool is very difficult to produce. The most feasible solution appears to be using an OWL based tool to output an OWL based representation of two different HL7 v3 models, and perform a comparison of the two OWL outputs to determine differences between the two models. It appears that an OWL tool has been developed by one of the developers that have a built-in tool to perform the OWL representation comparison. This is a work-in-progress tooling project. 2.8.2 CURRENT PROJECTS Currently there is no active Clinical Statement Project. 2.8.3 ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by Clinical Statement: Issue: This tool may be very useful for validating the conformance of patterns of detailed clinical models to CDA, as well as Pharmacy and Patient Care RMIMs. IT-014-06 and its OWL-based HL7 v3 model-model validator Action: It will be useful for IT14-06 subcommittees, NEHTA and PCEHR groups to keep a close watch on the development and maturation of this OWL based tool, and to evaluate its appropriateness and adequacy for adoption. subcommittees NEHTA Australia eHealth communities 50 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.9 COMMUNITY BASED COLLABORATIVE CARE 2.9.1 PROGRESS AT THIS MEETING As detailed below, the progress focussed on the collaborative work with the Security WG on the Data Segmentation for Privacy (DS4P) and associated projects. It is important that these work items are soundly and correctly integrated with respect to the current trials that are informing the projects. Details of these are given below and in the Security WG section. 2.9.2 CURRENT PROJECTS The following projects are reported on in this section: • Project #798—Refactor HL7 Confidentiality Concept Domain, code system value sets. HL7 Healthcare Privacy and Security Classification System, Release 1; • Project: Data Segmentation for Privacy (DS4P); and • Project #800 Behavior Health Domain Analysis Model, Messages and CDA Profiles (aka Behavioral Health Continuity of Care Document—BH CCD). 2.9.2.1 PROJECT #798: REFACTOR HL7 CONFIDENTIALITY CONCEPT DOMAIN, CODE SYSTEM VALUE SETS—HL7 HEALTHCARE PRIVACY AND SECURITY CLASSIFICATION SYSTEM, RELEASE 1 PROJECT OBJECTIVE The current HL7 Confidentiality concept domain, code system and value sets are overloading the coded attributes of confidentiality. As a result implementers are able to change the meaning of these attributes via the vocabulary bindings. The project provides a thorough analysis of the current confidentiality concept domains, coding system and value sets. As part of the analysis the CBCC WG intends to make clear a distinction in the confidentiality concept domain, coding system and value sets about how this vocabulary is to be used with patient-centric information. The project identifies the changes to be proposed that may include revised, deprecated and additional vocabulary to support and expand the current confidentiality codes. Further evaluation may also introduce additional concept domains, coding systems and value sets. The project also identifies the impacts of proposed changes on current implementations, communicates proposed changes to the community, and discusses and documents feedback. Based on the feedback this project may produce a Vocabulary Harmonisation Proposal. There are several projects under the parent project, as detailed below. Health Classification Scheme (HCS) This is new in healthcare, as no other standards currently exist for this and the project will produce a useful healthcare vocabulary for data tagging. It is developing a new scheme in healthcare originating from work in the US, based on the Data Segmentation for Privacy (DS4P) project that is an offshoot from the PCAST Congress of Health Information Technology report 2010. It was identified that gaps could be filled with a classification scheme, as the current coding is clinically based and does not include any social privacy or a security classification of this information. The Health Classification Scheme (HCS) looks at the perceived harm that could occur from the disclosure of this information beyond where it is used. It is policy based and builds on clinical objects in EHR but includes perceived risk of exposure. Therefore someone accessing the 51 Final Report HL7 Meeting—Atlanta, USA (May 2013) object (classification is a colouring on the clinical facts) needs to have appropriate access based on a security engine. The HL7 Security WG is working with the EHR WG in the area of data integrity to define the contextual differences in the meaning of integrity (e.g. security integrity means to protect against unauthorised modification, and for clinicians integrity relates to provenance, trustworthiness and history of the data). Personal Health Information Exchange—Privacy Information Content Substance Abuse and Mental Health SA (SAMHSA) may embark in a standardisation of vocabulary in regard to substance abuse data. This will include safety items, such as restricted access due to personal or community risk (e.g. violent behaviour) for certain policy reasons. It has been suggested that a step in the direction of detailed code subsets need to be put together (value sets), which can become a standard in HL7 and a vetted, open value set. If HL7 engages in this type of vetting on sensitive information it will become an open information resource. The project may be started in the SHARPS (Strategic Healthcare IT Advanced Research Projects on Security) program by Carl Gunther, which investigates how you take the indicators such as clinical semantic labelling and group in such a way to encapsulate (e.g. ‘this is the category’ for HIV), then apply access control system to these labels. PROJECT ACTIVITY/ISSUES AT THIS MEETING Health Classification Scheme (HCS) In the recent ballot 2% passed, 51 affirmed 11 over 15 negatives, and there were 57 abstentions and 27 no votes. There were a lot of abstentions, and it was thought that this was because it may not have been on the radar for vote as it was informative and not normative or voters were not aware of the work. Some of this may also have been due to the new HL7 rules where if a negative is recorded then a comment was required. Also this may have been driven by the new rule where getting involved in the derivate work is forced. If you are ever interested you MUST submit a vote (sign up and abstain). Alternatively it is possible that having that many abstentions means that people are interested, rather than just non-voting. These are issues that are a concern for any ballot. The result is that whilst the HCS was created because of the identified gap. This is part of the DS4P project, and as such with the HCS and HCS IG we will have two normative items and two informative in the package: HCS has two informative documents, which are— • one is explaining in the normative; and • the other is elaborating by example using the HL7 vocabulary. The DS4P items would be considered normative. The discussion of the format for the ballot was extensive, so as to assess the complications that would arise if the HCS and DS4P IG were balloted together. The concern was that the HCS would complicate the adoption of the DS4P internationally, as the HCS does not need to be adopted as is for the DS4P IG to function. The outcome was that the SEC/CBCC joint group would work on a new project proposal for the DS4P IG separately (this work is listed under the SEC WG as the sponsor of this work item). 2.9.2.2 PROJECT: DATA SEGMENTATION FOR PRIVACY (DS4P) Also see the new project under SEC WG. 52 Final Report HL7 Meeting—Atlanta, USA (May 2013) PROJECT OBJECTIVE The term ‘data segmentation’ refers to the process of sequestering from capture, access or views certain data elements that are perceived by a legal entity, institution, organisation or individual as being undesirable to share. This basic definition does not however account for the multiple permutations of segmentation in the healthcare context (e.g. granularity) nor does it adequately capture the varied considerations required for development of segmentation policy. A US national project called ‘The Data Segmentation for Privacy Initiative (DS4P)’ is based on the Data Segmentation for Electronic Health Information Exchange Whitepaper from ONC, produced to support the S&I Framework. Additional information can be located at the S&I Framework wiki (http://wiki.siframework.org). The S&I Framework is to enable the implementation and management of disclosure policies that originate from the patient, the law or an organisation in an interoperable manner within an electronic health information exchange environment, so that individually identifiable health information may be appropriately shared for:— • patient treatment and care coordination; • third party payment; • analysis and reporting for operations, utilisation, access quality and outcomes; • public health reporting; and • population health, technology assessment and research. The purpose of this initiative is to enable the implementation and management of varying disclosure policies for an electronic health information exchange environment in an interoperable manner, with the goal of producing a pilot project allowing providers to share portions of an electronic medical record while not sharing others, such as information related to substance abuse treatment (which is given heightened protection under the law). Data segmentation—also referred to as data tagging, data labelling and metadata tagging—is important to security as it relates to privacy metadata and the issue of interpretation of the confidentiality code between organisations. The initiative is to address issues of determination of which part of the health record should be protected by jurisdictional or organisational policy or by regulation (e.g. substance abuse info and vet affairs providers for three specific conditions). For example if you have a document that has protected information and non-required protection, how does a system identify and manage this? Also, what occurs when this information is passed on to another organisation? How do you define this for the receiving organisation (i.e. using the metadata used in the transportation of information)? On a national basis the progress to date has been to identify use cases, and now scoping requirements are being undertaken. PROJECT ACTIVITY/ISSUES AT THIS MEETING Update on progress of S&I Framework progress in relation to DS4P is that upon a recommendation to the US HITSC Privacy and Security Work group in March, the Data Segmentation for Privacy (DS4P) Initiative has transitioned from a fully facilitated initiative to a community led initiative. This is significant in its aim to integrate into the wider healthcare environment and for full adoption. Deliverables from the Initiative include the DS4P Use Case, three implementation guides, test procedures, and evaluations and completed requirements traceability matrices from the completed pilots. Future work in the initiative will focus on implementation and testing, and transition of the DS4P implementation guides to standards development organisations including HL7 for refinement and maintenance. Five pilots are underway for the initiative, 53 Final Report HL7 Meeting—Atlanta, USA (May 2013) including VA/SAMHSA, the Software and Technology Vendors Association (SATVA), NETSMART, Jericho Systems/UT-Austin, and Greater New Orleans Health Information Exchange (GNOHIE) pilots. Pilot activities are based on the Reference Implementation Guide, describing the use of standards for applying privacy metadata to enable the sequestering of certain data which requires enhanced protection under the law, while allowing other data to flow more freely. The S&I Framework Working Group have initiated an implementation guide (IG) for the DS4P work. They have requested to transfer the IG publication to the standards organization to align and maintain them in an appropriate way. IHE, OASIS, HL7 are delighted to get response from all the SDOs but appreciate that HL7 Security and HL7 CBCC are working collectively to work on the DS4P for direct and exchange project. S&I will continue to support the effort. S&I report that it is it has been concluded that the national initiative need the 'go with the national initiative’ to be vetted through the SDO process. Although there is considerable participation, S&I still want to be involved in the SDO process. It is proposed that the involvement of the community in the IG and its use in the DS4P will be an exemplar that needs input from the SDO community. Currently there is a web service example—an SMTP based IG and another Restful or FHIR example in trial— and these are the intended three modes of transport that S&I are looking to implement. The SEC and CBCC WGs will need to devise the ballot process. The RESTful implementation will need collaboration with IHE. There are five pilots up and running and two more under development. The US Veteran Affairs project is moving into systems implementation and is no longer a pilot. CBCC member Iona S in conjunction with SAMHSA have worked on the test procedures for the implementation and successfully completed the testing that needs to be done. The testing scripts are based on the user stories and they have been tested against the pilot. This pilot is aimed at a high level so that all the use cases can be captured. This will add to the completeness of the IG and add to the conformance measures. The issues discussed were about the development of this work as an international standard, and not just for the US realm. It was decided that we need to produce the US guide first. After this we will produce the EU profile, as this includes the classification types we can move to a wider application. Also careful consideration needs to be made of the differing legal aspects. It was agreed that the work should aim to be international, particularly as we refer to some of the ISO specifications (e.g. 26 000) and using labelling as an approach seems the most effective for the future. ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by CBCC/Security: Issue: Increased involvement of Australia in input/feedback on HCS as a foundation for information sensitivity labelling and secure handling. SA Healthcare Classification Scheme (HCS) Action: Notification by SA to IT-014-04 balloting of HCS, and IT-01404 to review and comment on this to provide feedback to HL7 Australia on next ballot. IT-014-04 54 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.9.2.3 PROJECT # 800 BEHAVIOR HEALTH DOMAIN ANALYSIS MODEL, MESSAGES, AND CDA PROFILES (AKA BEHAVIORAL HEALTH CONTINUITY OF CARE DOCUMENT (BH CCD) PROJECT OBJECTIVE HL7 has several standards that support the interoperability requirements of the behavioural health community, including the HL7 EHR Behavioral Health Functional Profile, Release 1, the HL7 Social Service Invoice Common Message Element Model (CMET), A_BillableSocialService universal (COCT_RM610000UV06),the Semantic Health Information Performance and Privacy Standard Domain Analysis Model (SHIPPS) and the Consent Directive Clinical Document Architecture (CD) Implementation Guide Draft Standard for Trial Use (DSTU). These standards were developed over several years with input from a wide range of behavioural health experts from the US and abroad. Some stakeholders have more encompassing requirements than this current set of standards supports. PROJECT ACTIVITY/ISSUES AT THIS MEETING The project was a 2013 May Ballot Cycle Info: DSTU Ballot as HL7 Implementation Guide for CDA® Release 2: Behavioral Health Assessment, Release 1—US Realm NIB, and an Informative Ballot for HL7 Version 3 Domain Analysis Model: Behavioral Health Assessment, Release 1—US Realm NIB. Behavioural health DAM is specific to behavioural health. It was used as a basis for analysis for CDA2 and creating the privacy consent directive CDSA IG went for normative ballot. When there is resolution of the two organisational negatives the ballot will pass. The aim (in terms of process) is to create a DAM from a CDA document. To date this has been using the open health tools and created java libraries for the privacy consent directs. ACTIONS FOR AUSTRALIA No actions required at present. 2.9.3 2.9.3.1 OTHER NON-PROJECT WORKING GROUP DISCUSSIONS AND PRESENTATIONS PRIVACY CONSENT (CONSENT TO SHARE) An example of a common sense privacy consent/policy choice model is given below: A Common Sense Privacy Consent/Policy Choice Algorithm Purpose: To describe an intuitive process to segment and protect privacy sensitive, clinical information, so that patients can knowingly choose more or less protection (1) Stigmatised/sensitive Conditions? a. No; then no restrictions; STOP b. Yes; then proceed (2) Sensitive condition/problem ontology, initial use cases a. Substance abuse, by substance type ontology b. Mental health problems by problem ontology 55 Final Report HL7 Meeting—Atlanta, USA (May 2013) c. Sexually transmitted diseases (STDs), by type d. HIV/AIDS (3) Threats/risks/costs due to unauthorized disclosure of clinical information a. Legal (e.g. loss of parental rights, criminal justice) b. Financial (e.g. employment opportunities, life insurance) c. Social status, position in communities and family (4) Levels of trust, as defined by giving access to sensitive information a. ‘Care Team’ or ‘Core Care Team’ i. If no concerns, then no restrictions; STOP b. If some concerns, then some restrictions; then proceed to V i. If grave concerns, then global restriction: then proceed to VI c. Extended Care Team (see i, ii, iii above) d. Other Third Parties (see i, ii, iii above) (5) Record segmentation gradients/staging process a. Only hint at issues, do not disclose detailed problems (e.g. indicate addiction symptoms without disclosing specific substances abused) b. Only disclose less sensitive problems (e.g. show alcohol but hide heroin use) c. Underplay problem severity/number of symptoms (e.g. show past use, but not current) d. Global restrictions (6) Reconsider privacy policy restrictions (Step V) if/when a. Restrictions create personal safety risks b. Payers can sidestep privacy restrictions and share data with third parties c. Jurisdictions require reporting public safety risks. Healthcare organisations that deal primarily with sensitive information such as SAMHSA have a complex mix of requirements that people will have to know in order specific privacy consent. It is designed to provide a personal user-interface which gives people a series of choices as to who their information is sent to, to the main point at some point (previous controversial) detailed. In a primary care record some of the information is interspersed within the record. Essentially this consideration is trying to be forward looking and giving accountability to the organization and giving some additional privacy protection and specifying more or less privacy. The requirements include the following: • An ability to filter the information. • Sanitise the information, so that the risk of being stigmatised is minimised. • Subsequent healthcare providers may want to ask some questions and this may then expose the information that was intended to keep private. This is also in relation to information being sent to central repositories, such as defined by the US PCAST, and similarly the Australian PCEHR. • This will rely on the clinical rules engine and policy rules engine allowing, at various levels of granularity, to segment and send information in a restrictive manner. Using standard terminology this is attempting to define the rules in terms of levels of trust and risk, and segmenting the record accordingly. This avoids the ‘all or nothing’ approach. This approach is trying to 56 Final Report HL7 Meeting—Atlanta, USA (May 2013) simplify the healthcare providers managing the consent and information sharing, and providing a more granular consent, so that people do not have to make the choices—often incorrectly. In the US this also related to the financial status of the patient or payer. What is important is to weigh the interest of the patient clinically and health wise against ‘need to know’ consent (e.g. a dermatologist requesting mental health information). In Australia, when information for instance on patient referral is provided to specialist, how much of the history that is not relevant is provided? This may not be related to hiding information but protecting the patient’s feelings about the information. In the US this also relates to the ‘payer’, however all countries need to consider the national policy coverage, local coverage determination which indicates the information needed to justify a condition. ACTIONS FOR AUSTRALIA No actions required at present. 57 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.10 2.10.1 EDUCATION WORKING GROUP AND MARKETING COMMITTEE PROGRESS AT THIS MEETING The strong focus on education in HL7 was again evident this WGM. There were a number of important areas considered including the educational requirements for FHIR and the progression of the e-learning agenda. The impact of the changes to IP availability were again raised in the context of education being one of the key member value propositions for HL7, as well as leveraging new developments such as FHIR. Potential resurrection of the certification project was suggested in several discussion forums and it was suggested that HL7 Australia could lead this project. The role of HL7 International paid positions at headquarters was discussed, as input and support from such positions will likely become increasingly important as education is featured more prominently in business and membership models. Potential education platforms were outlined as: e-learning, webinars, face to face workshops, hands on at connectathons, ambassador presentations and affiliate programs. In addition to these topics there was a presentation from the International Mentoring Committee that is continuing to target Africa and low and middle income countries (LMIC) for the development of standards and education to develop local standards experts. Two delegates from Kenya were in attendance at the WGM. The eLearning program will be open to LMIC with the objective that academics from such countries may take up these educational opportunities to foster local development and understanding. The International Mentoring Committee proposed the possible topic for the September plenary meeting as ‘low and middle income countries access and usability’. Planning for the Boston WGM included leaving the meeting schedule unchanged with joint meetings with marketing and international mentoring, again held in the Monday lunchtime session and the quarter one and two meeting spaces for project work and planning. 2.10.1.1 MARKETING AND EDUCATION REQUIREMENTS FOR FHIR A strong methodological and dynamic approach was discussed as necessary in relation to FHIR. Education is needed around developer conformance to achieve a high degree of consistency. The education strategy needs to be targeted at both entry level and more proficient users (base level and more advanced). Emphasis was given to the fact that FHIR is new and changing and the strategy needs to reflect this. Education also needs to be evolving and able to match updates from the development team. The importance of feeding in updates and change needs directly and timely to the education WG was highlighted. The suggestion was made that rather than having to be so responsive a large part of the focus and strategy could be to develop the knowledge base around where to find content and read changes. The requirements for practical and sample implementation was also discussed e.g. how to start communicating in FHIR, how the governance works, how the management group works, where to find attributes. This could all be conveyed via a light weight webinar or pre-recorded meeting. Potential audiences include implementers, decision makers/governments, standards organisation/people, technology and application architects (vendors). Marketing to these groups needs to include consideration to the key questions: what is FHIR about and why should I care? What is it about what HL7 is doing that makes me want to pursue it? The suggestion of the need for a market analysis was made. The strong point that FHIR is not like other aspects of HL7 was made and that there is an inherent risk that that the people who will use it won't be drawn effectively into the HL7 membership community. Being effective in the use of FHIR was suggested as a necessary benefit of membership. The critical question therefore becomes how does HL7 International convey and communicate this effectively? 58 Final Report HL7 Meeting—Atlanta, USA (May 2013) Other key strategic issues and questions include: • Leveraging projects that are using FHIR • Positioning of FHIR and how it reinforces the HL7 brand (e.g. mobile health) Potential limitations were recorded as: • Community that will get the most use out of it are likely to never be represented in the HL7. Also the potential implication that healthcare IT is simple and easy may be conveyed. • Education needs to be linked strongly to marketing and from these discussions, it can be concluded that education needs to be linked to membership as well. 2.10.2 CURRENT PROJECTS The following projects are reported on in this section: • Project #865: HL7 Certification Program Enhancement • Project #548: Strengthening HL7 Education (Education Strategic Plan) • Project #877: Develop HL7 Ballot Site Education Tutorial 2.10.2.1 PROJECT #865: HL7 CERTIFICATION PROGRAM ENHANCEMENT PROJECT OBJECTIVE This project will enhance the HL7 certification program by taking into account the process used for certification as well as the actual certification testing. Enhancements include: • other HL7 International Educational opportunities e.g. e-Learning, HL7 International Summits or WGM tutorials; • educational events organised by HL7 Affiliates or other organisations e.g. workshops, conferences, elearning, etc.; • the certification candidates work experience with HL7 products; and • the certification candidate involvement in HL7 International or Affiliate WGs e.g. Co-Chair, member, etc. The certification program will also take into account the roles and skill levels needed to work with HL7 standards in healthcare-related organisations at different areas of responsibility. The project will: • establish a table of competencies categories, subcategories and skills, related to HL7 standards or products; • establish areas of responsibility/job roles related (using, developing, making decisions) to HL7 standards/products; • establish a framework, mapping the roles to the competencies; • define the name of the levels of certification; • define and/or investigate existing skill metrics (i.e. how to acquire credits for certification) and create the policies; 59 Final Report HL7 Meeting—Atlanta, USA (May 2013) • evaluate the current HL7 and HL7 Affiliate offerings in terms of specific skills acquisition and create the corresponding Quality Assurance mechanisms; • define the process for the certification program management e.g. HL7 staff chores, HL7 Affiliate chores (options include certifying people and/or a course) and define the process for post-certification continuing education (CE); and • launch the new certification program. PROJECT ACTIVITY/ISSUES AT THIS MEETING Some discussion was dedicated to this project at the current WGM. Issues discussed included electronic certification and home proctoring regulations. Concerns have been expressed by some affiliates about the use of “home proctoring” where certification candidates sit exams on their home computer with a web cam for monitoring and the computer locked down to prohibit access to exam content. Some further thought and planning will be undertaken in this area. Discussion amongst the Australian delegation was positive in developing some extended credentialing for practitioner certification and this will be picked up locally via the HL7 Australia Board and Education Council. HL7 International Director of Education, Sharon Chaplock, also expressed that the electronic bank of exam questions will help to guide and inform a blueprint of competencies for certification levels. 2.10.2.2 PROJECT #548: STRENGTHENING HL7 EDUCATION (EDUCATION STRATEGIC PLAN) PROJECT OBJECTIVE This project aims to develop a policy by which affiliates are encouraged to advertise HL7-wide educational offerings such as e-learning, as well as notifying HL7 HQ of their own educational initiatives so that they can be added to the HL7-wide calendar of educational offerings. It will define 'associates' and determine appropriate means and policies for interacting with associates. Distinct policies might be crafted for different classes of associates. PROJECT ACTIVITY/ISSUES AT THIS MEETING Heather Grain is currently developing a webinar on how to develop a HL7 tutorial following her review of all of the HL7 tutorial content and framework. A matrix has been developed that outlines important information about each HL7 tutorial including: audience, product family, tutors, objectives, level (e.g., beginner, intermediate, advanced etc). The vision for this is to roll out for each work group starting with one trial WG (e.g. this is underway with Tooling and was suggested for use with FHIR). This will be reviewed by David Hay (NZ) for FHIR requirements. Heather Grain will also be involved in the development of a UML tutorial specification. This will possibly be available at the January 2014 WGM (at the earliest). SAIF tutorial has been dropped from the tutorials list due to low registrations. Requirements have been added by the TSC for tutorials and there will be a webinar in response to this. 2.10.2.3 PROJECT #877: DEVELOP HL7 BALLOT SITE EDUCATION TUTORIAL PROJECT OBJECTIVE The intent of this project is to develop a tutorial on how to use the HL7 Ballot site. This tutorial will be developed in English. 60 Final Report HL7 Meeting—Atlanta, USA (May 2013) PROJECT ACTIVITY/ISSUES AT THIS MEETING No update available from this WGM. 2.10.3 OTHER NON-PROJECT WORKGROUP DISCUSSIONS AND PRESENTATIONS HL7 FUNDAMENTALS (PREVIOUSLY THE E-LEARNING PROGRAM) 2.10.3.1 As Heather Grain previously reported in the January 2013 Phoenix WGM report, “The e-learning project has more than 600 students who have registered. Materials have recently been updated and the examination components are now automatically marked. A site has been established where tutors can suggest changes. The course is now called HL7 Fundamentals, rather than the e-learning course. Within the current WGM access to the e-learning materials has been achieved and electronic copies of materials will be forwarded to HL7 Australia’s Education Council. A FHIR component will be added and this has been developed in conjunction with David Hay (NZ). The suggestion was made that XML and UML are mandatory pre-requisite knowledge for entry to the course. HL7 Brazil presented a summary of their e-learning course. The course is 15 weeks with XML and XML mandatory (these can be completed in one additional week each). The course is priced at $25,000 USD and has a 75% average course requirement to receive certification and fulfil the requirements for successful completion. The course has around an 8.8 hours per week work requirement however there were indications that this may be higher overall at around 10 to 20 hours. A total of 92% of enrolments had no experience in HL7. The course leverages online forums for tutors, online chat space for student to student and student to tutor (which is reportedly well used by students) and also uses social media groups. New Zealand has run the e-learning course once with less than favourable results. This evaluation was consistent in Canada and this was primarily due to material concerns as some were seen to be inappropriate. US feedback was that student frustration and annoyance increased from Version 2 to Version 3 and so too did the time requirement for the course during this learning component. 2.10.4 ACTIONS FOR AUSTRALIA Topic Issue / Action / Recommendations for Australia Recommended for Action by Education: Issue: There is agreed and inherent value in building varying levels of certification for an advanced practitioner level as distinct from base level certification. Previously a certification project was active with the Education Work Group however the project leader was no longer available and the project is no longer active. HL7 Australia Action: HL7 Australia to consider taking up leadership of this project to reinstate as an active project. WGMs Issue: An e-learning course for Australia could be established. The course can be set up for the HL7 Australia Board to review. (This issue was reported by Heather Grain at the Phoenix 2013 WGM and continues to be relevant.) HL7 Australia Certification Project Education: e-learning Plan Future Education representative delegations to Education Council Action: HL7 Australia Education Council to review e-learning course materials. 61 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.11 ELECTRONIC HEALTH RECORDS 2.11.1 PROGRESS AT THIS MEETING Most of the EHR WG activity at this meeting involved: • Reconciliation of ballot comments received on the flagship standard, EHR System Functional Model Release 2 (EHR-S FM R2) that is also being progressed in parallel as an update of ISO/HL7 10781 and as a JIC-endorsed project. • Reconciliation of ballot comments on the work item to elevate Personal Health Record System Functional Model from DSTU to a normative ANSI/HL7 standard, being balloted in parallel in ISO/TC 215 as ISO/HL7 16527 as a JIC-endorsed project. The EHR WG also confirmed that the following two projects are currently active and resourced (both are discussed in more detail below): • Project #688: EHR System Function and Information Model (EHR-S FIM). • Project #831: EHR-S FM Profiling tool It was noted that the following projects have been moved to inactive status because of completion, being rolled into other projects, or no longer being resourced. • Project #843: CDA Transform to Blue Button: The project is finished and published. “Blue button” functionality enables information to be extracted from clinical information systems in a portable format meeting US-Government requirements. • Project #819: EHR-S Profile for Health Information Exchange Metadata - this project went to commentonly ballot for May 2012 with outcomes taken into account for EHR-S FM R2. • Project #739: HL7 EHR FM Records Management and Evidentiary Support Functional Profile Implementation Guidance - Phase 1 - Environmental Scan and Analysis - this project went to commentonly ballot in 2011 with outcomes taken into account for EHR-S FM R2 and pending update of RMES. These three projects were particularly oriented towards the needs of the US-realm. Other points of note included the following: • Project #1008: Meaningful Use EHR System Functional Profile (of EHR System Functional Model Release 2). [See further report below]. • Preliminary discussion of the form that the future Release 3 of the EHR System Functional Model might take. How to move forward? Steve Hufnagel spoke to a 16 page outline and call for volunteers to work on a fundamental reengineering of the next generation of the EHR-S FM from a normative framework populated with some 320 functions and 2300+ conformance criteria to a reference architecture: “EHR-S Functionand-Information Reference Architecture Release 3.0 (EHR-S FIM RA-3.0)” in which the specific verbs and criteria would be in profiles and not the architecture itself and the architecture would identify functions in terms of the information objects they process. A parallel vision suggested by Helen Stevens is of a library of functions, assembled and constrained by various functional profiles as required. More work is required on refining and agreeing the concepts. The topic is to be progressed on fortnightly teleconferences (of the EHR Interoperability Sub-Group) with a view to creating a two62 Final Report HL7 Meeting—Atlanta, USA (May 2013) year Plan of Actions and Milestones (POA&M) and Project Scope Statement (PSS) for approval at the next HL7 WGM in September 2013. [Also see comments under Project #688: EHR-S FIM, concerning this project’s influence on development of R3 of the jointly balloted and accepted EHR-S FM. This influence needs to be monitored and, if possible, guided to ensure that the outcomes do not prejudice the ability to continue future versions of ISO/HL7 10781 as an international EHR-S FM standard]. • Project #995: Usability Guidelines for EHR Systems. The title for this project in the current project list (below) has been updated and a project progress report provided. • Project #984: Reaffirmation of HL7 EHR Behavioral Health Profile, Release 1, and • Project #985¨ Reaffirmation of normative standard for HL7 EHR Child Health Functional Profile, Release 1 The functional profiles addressed by these two projects are ANSI/HL7 normative specifications which will hit the 5 year mark for systematic review at the end of 2013. These will be included in the September 2013 ballot for reaffirmation. Given changes in the EHR-S FM since the documents were first published and parallel work in on R2 profiles for the Behavioral Health (BH) and Child Health (CH) domains, explanatory material will be included outlining the status and differences in content of R1, R1.1 and R2 of the EHR-S FM (i.e.) and pointing to continuing work on providing profiles against R2 to address current needs of the BH and CH domains. • Proposed reinstatement of: Project #610: HL7 White Paper: 'Balancing the Integrity and Value of Personal Health Records with...' (Aka “The PHR White Paper”). The full title of this document, originally produced in 2009, is: "Balancing the Integrity and Value of Personal Health Records with Consumer Adoption, Current Health Information Technology Standards, and Privacy Rights – A Discussion of the Alteration of Professionally-Sourced Data". This 35-page document was prepared to provide background and research needed to complete the PHR-S FM and it was used to inform its content. It was noted that the PHR domain has changed since 2009 and the White Paper should to be updated to take into account Mobile Health, Bring-Your-Own-Device, Blue Button, and legal/governance, other work that has occurred in the industry and changes in the stakeholder population. A new set of volunteers who could help update the white paper are to be invited, including: Professional associations representing risk managers, fraud prevention, and lawyers, and the BYOD technology advocates. • Project #982: EHR-S Public Health Functional Profile Release 2 - Reconciliation of information only ballot. • Joint meeting with Mobile Health hosted by EHR WG. Elysa Jones of OASIS gave a brief presentation seeking the level of EHR WG interest collaboration on PHER WG projects: - Project #971: Implementation Guide for transforming messages between OASIS Tracking of Emergency Patients (TEP) and HL7 V3 63 Final Report HL7 Meeting—Atlanta, USA (May 2013) - Project #970: Implementation Guide for transforming messages between OASIS Tracking of Emergency Patients (TEP) and HL7 V2.7.1 The main focus of the session was a presentation by Tim McKay of the mHealth WG on how mobile devices are getting more and more important in healthcare organisations and applications but HL7 standards are slow to recognise this. Key points arising were: - The basic framework of PHR-S FM doesn’t have to be changed for mobile devices but some functionality needs to be tailored for the new capabilities offered by mobile devices (such as geo-location, app-controlled cameras, environmental and motion sensors, and SMS messaging). - mHealth WG suggest that some profiles be created for the PHR-S FM that account for use of mobile devices and that the information obtained also be used to inform other HL7 work groups of where they might be affected by mobile device usage. - Mobile health applications have the potential to cut across many of the Meaningful Use (MU) criteria in the US and the standards that support them, specifically in the areas of: messaging, document architecture, functional models and services. This needs to be considered in relation to the S&I framework, to optimise mHealth support for MU. • Joint meeting with Security and CBCC WGs hosted by EHR WG. Discussion in this session focused on issues arising from work on R2 of the RMES functional profile including the implications of the many new requirements for EHR systems to hold metadata on record entries in EHRS FM R2 (and they are extensive). Security WG has a strong interest in having requirements for Privacy and Security metadata that support its Healthcare Classification System (HCS) The RMES Sub-group and Security WG also see work on EHR-S FM R2 as a continuing opportunity to further establish commonality in key terms and concepts that will serve other Work Groups. [NB. These changes will probably result in further Glossary changes needing to be tracked and harmonised]. • Joint meeting with PC, PHER and Pharmacy WGs and Clinical Interoperability Council (CIC) hosted by EHR WG. This was basically an update session in which presentations were given on the following topics: - Public Health Functional Profile team’s experiences updating EHR-S FM R1 Functional Profiles to the new EHR-S FM Release 2 format (John Ritter). - Work on Project #1004: V3 Allergy and Intolerance Clinical Models being led by the Patient Care WG on (Elaine Ayres) - Patient Care WG work on developing standards for Care Plan (Stephen Chu) - Work by the CIC to support the (US-realm) S&I Framework Structured Data Capture initiative, which uses the "Retrieve Form for Data Capture" (RFD) for public health data. • Joint meeting with FHIR, SOA, Pharmacy, Security hosted by EHR WG (Thursday Q2). The session commenced with two presentations were given by John MoehrkeI in the nature of a “Free Security Tutorial”. They are available from the HL7.org web site at: - May 2013 Security Education: Audit Logging and Reporting - May 2013 Security Education: Sec, mHealth and FHIR 64 Final Report HL7 Meeting—Atlanta, USA (May 2013) Subsequent discussion centred on the need for some EHR-S FM conformance criteria (specifically, the Record Lifecycle event metadata in R2) to be accommodated within the FHIR framework, noting that this harmonization effort needs to occur by July 2013. FHIR tags need to begin envisioning the workflow and processes related to information exchange, in particular: Access Control and Audit Event Reporting. Australia had difficulty maintaining a presence in the EHR WG activities during the week as the WG runs solidly throughout the week and there were many schedule conflicts. Meaningful engagement in ballot reconciliation was also difficult because much of this is extremely detailed and is done in small break-out teams and Australian delegates, when available, could only contribute from their knowledge to a single stream at a time. Australian contributions are always welcomed by the EHR WG and we have a long history of making modest but worthwhile contributions to these standards, which are primarily still used in the United States, although there has also been some significant use in several other countries. 2.11.2 CURRENT PROJECTS The following projects are reported on in this section: • Project #551: EHR System Functional Model (EHR-S FM) Release 2 • Project #660: PHR System Functional Model (PHR-S FM) • Project #688: EHR System Function and Information Model (EHR-S FIM) • Project #831: EHR-S FM Profiling tool • Project: EHR FM R2 Glossary Update. Completed; remove from list for September 2013 report • Project #995: Usability Guidelines for EHR Systems – project information updated • Project #1008: Meaningful Use EHR System Functional Profile (of EHR System Functional Model Release 2) 2.11.2.1 PROJECT #551: EHR SYSTEM FUNCTIONAL MODEL R2 PROJECT OBJECTIVE The EHR System Functional Model (EHR-S FM) provides a consistent framework of functional capabilities against which the functionality of an EHR system may be evaluated. It is a comprehensive list designed to be used with functional profiles, each of which identifies which of the many capabilities are required in a particular setting (e.g. primary care, aged care or acute care emergency department). Release 1.1 was published as a full ANSI normative standard and also as the international standard, ISO/HL7 10781:2009 Health Informatics, Electronic Health Record System Functional Model Release 1.1. Key objectives of the current EHR-S FM R2 project are to: • enhance the current joint ISO/HL7 EHR-S FM Release 1.1 into Release 2.0 incorporating input from functional profiles, CCHIT certification criteria, EHR Interoperability Model DSTU and EHR Life Cycle Model DSTU, plus additional content recommended and developed as a result of the R1.1 joint ISO/HL7 ballot comments; and 65 Final Report HL7 Meeting—Atlanta, USA (May 2013) • To support EHR-S FM R2's users' needs to quickly produce Functional Profiles that are conformant to base EHR-S FM Conformance Clause, tooling may be developed that includes an XML version of the EHR-S FM R2. Redevelopment of existing profiles (subsets) derived from the EHR-S FM in various care settings e.g., long term care, child health, emergency services is not included in this project but are the subject of separate consideration. Developers of existing profiles will be encouraged to update the profile based on the new R2 development and many already have a strong need to update to R2 but cannot do so with certainty until the updated standard is available. PROJECT ACTIVITY/ISSUES AT THIS MEETING This project is being progressed as a JIC Joint Initiative with parallel balloting in ISO/TC 215, CEN/TC 251, GS1 and in HL7 International. Previous reports had noted that: • The first HL7 normative ballot of the R2 text closed on 7 May 2012 with 866 comments to be addressed. • The parallel ISO/TC 215 DIS ballot did not close until 26 September 2012 but passed with 100% of the vote and around 40 comments needing to be reconciled (some containing sub-comments). The following points were noted from discussion at this WGM: • Following the previous WGM, a further updated draft had been prepared as planned with the second normative (HL7/N2) ballot opening on 25 March and closing on 29 April 2013. • 395 comments were received in the latest HL7/N2 ballot, including some negative majors. Taking into account the earlier ISO/DIS and other SDO ballots, there will have been over 630 changes since the original ISO/DIS draft was issued. • Reconciliation of the HL7/N2 ballot comments had commenced via teleconferences and individual offline reviews and continued through breakout groups at this WGM. Significant progress in ballot reconciliation was achieved; however, there were around 100 items remaining at the end of the WGM to be resolved over the coming weeks. Completion was expected by mid-June. • All ballots had passed, but because of the number of substantive changes, a further ISO/DIS2 and HL7/normative ballot will be required. • It is the view of the Co-Chairs that the reconciliation has not resulted in any “substantial” changes from HL7’s point of view and that the next HL7 ballot would be a final confirmatory ballot limited to those who had voted in the previous N2 ballot. • Some requested changes that might have been seen as substantial were considered an extension of the R2 scope and were deferred for consideration in the next release (possibly a release 2.1) • Harmonisation of these ballots is posing a challenge because of the different timescales and rules for voting and commenting on second round ballots in ISO, CEN and HL7 International. As JIC Chair, Richard Dixon Hughes has been asked to assist in resolving these issues in collaboration with the EHR WG Co-Chairs, Naomi Ryan (ISO/TC 215/WG 1 secretariat) and Lisa Spellman (ISO/TC 215 secretariat). • Significant applications of the EHR-S FM have been reported in: Ireland, Brazil, Mexico (2010), USA (extensively through CCHIT), The Netherlands (for Behavioural Health), Japan, Italy and Canada. 66 Final Report HL7 Meeting—Atlanta, USA (May 2013) • Thanks to the efforts of Lisa Spellman (ISO/TC 215 and JIC secretariats) and Dr Mary Lou Pélaprat (ISO/CS), previous issues surrounding the ISO publication formats for the EHR-S FM appear to have been resolved. 2.11.2.2 PROJECT #660: PHR SYSTEM FUNCTIONAL MODEL (PHR-S FM) PROJECT OBJECTIVE The full title of this project is, ‘HL7 Personal Health Record System Functional Model - Promote from DSTU to Normative and Promote to ISO TC215 under ISO/HL7 Pilot Agreement’. The background to this project is as follows: • The HL7 PHR-S FM specification was published by HL7 as a draft standard for trial use (DSTU) in 2008. • A profile was also developed to specify the functions of a shared PHR system that includes many functions similar to those that might be considered relevant in the context of the Australian PCEHR project. • Following expiry of the trial use period, the document is being updated and balloted as a full ANSI/HL7 normative standard. The most recent release is a major update. • It is also being jointly balloted as a full international standard, ISO/HL7 16527 as a JIC Joint Initiative. This standard addresses the functional needs of Personal Health Record (PHR) system developers and users. PHR information is expected to be sent, received, or exchanged from multiple systems, including: EHR systems; insurer systems; payer systems; health information exchanges; public health systems; Internetbased health education sites; clinical trials systems; and/or collaborative care systems. Work is being harmonised as much as possible with work on R2 of the EHR-S FM. PROJECT ACTIVITY/ISSUES AT THIS MEETING The following points were noted from discussion at this WGM: • Within HL7, the first normative (HL7/N1) ballot closed on 24 September 2012 and reconciliation within HL7 has been underway from that time onward. In total, there are some 318 comments to be reconciled from the HL7 ballot. • The ISO/TC 215 DIS ballot closed on 5 February 2013 and was approved with 17 in favour, one negative (UK) and 13 abstentions – there were 36 comments from ISO/TC 215 respondents. • The attendees at the April TC 215 meeting in Mexico City resolved to allow PHR-S FM to a second round ISO/DIS2 ballot once ballot reconciliation had been completed within HL7. • The UK voted negative on the PHR-S FM ballot based on the immaturity of the PHR system market in the UK and suggested that the document be offered as an ISO technical report, instead. Norway had suggested that it would have preferred that it be a technical specification. • Applications of the PHR-S FM have been noted in: UK (Telehealth), Wales (Welsh Care Record Service) and USA (county PHR systems). Given the reports of use in the UK, it is interesting that the UK responded negatively on the grounds that the market for was not sufficiently mature. The comments in the ballot did not appear to reflect the UK usage. A small team met throughout the week to work to reconcile the PHR-S FM ballot comments, while a larger group focussed on the EHR-S FM. The PHR group will continue its efforts by teleconference with expected completion by mid-June. 67 Final Report HL7 Meeting—Atlanta, USA (May 2013) The next step will be the joint HL7/N2 and ISI/DIS2 re-ballot, which should be ready to open soon. [Australian interests needs to be ready to review and respond to this invitation, noting that it is an N2/DIS2 ballot and that responsibility for this work within IT-014 is moving from the former IT-014-09 to IT-014-13.] HL7 will re-cast the document into the same format as EHR-S FM R2 prior to submission for HL7/N2 and ISO/DIS2 ballot. This should avoid excessive delay in getting the ballot documents issued by ISO/CS. 2.11.2.3 PROJECT #688: EHR SYSTEM FUNCTION AND INFORMATION MODEL (EHR-S FIM) PROJECT OBJECTIVE This project is described as producing a set of Conceptual Information Models called EHR-S ‘data profiles’. Each EHR-S data profile corresponds directly with an EHR-S function profile. Pairs of EHR-S function profiles and data profiles can be used to define business objects that can be composed into software components, capabilities, applications, systems and their information exchanges e.g. messages, documents and/or services. The superset of EHR-S data profiles is called the EHR-S Information-Model. The objective of this project is articulated by its originators, Steve Hufnagel and Nancy Orvis, as: “The EHR-S FIM vision is that analysts, engineers or testers can efficiently compose and refine the architecture and workflow agnostic EHR-S FIM into interoperability specifications, which meet their System Acquisition, Implementation or Test needs.” PROJECT ACTIVITY/ISSUES AT THIS MEETING As previously noted, even though this project has been underway for some time, a lot of the proposed detail of the FIM (Function and Information Model) has yet to emerge, despite some specific areas having been developed in detail to illustrate the concepts. There was no explicit discussion at this meeting focussed on progressing the FIM as a separate project. It appears that the energies of the FIM team (including their fortnightly teleconference calls) are now fully deployed in developing proposals to generalise and re-shape the next major release (Release 3) of the EHR-S FM to effectively be the FIM. The FIM work is strongly based on the interests of some in the US Military and with all the use cases and comparisons focussed on the need of national programs in the US realm. There has also been some presumption as to adoption of the HL7 v3 RIM as the underlying reference model for the information aspects, although this may have been moderated. Australian needs to continue monitoring this development and, if possible, contributing constructive input to ensure that any evolution of EHR-S FM is appropriate to it’s continuing to be a jointly developed and maintained international standard. 2.11.2.4 PROJECT #831: EHR-S FM PROFILING TOOL PROJECT OBJECTIVE This project will produce a (web-based and/or desktop) tool to create EHR-S FM profiles (starting with the EHR-S FM R2), with enforced profiling rules, and exports as documents, support for an XML interchange format for reuse across profile tool instances or for use in other tools. Additionally, the tool aims to support multiple platforms, Windows, Linux, and MacOS and to be available via OHT. There is a defined XML exchange format that is used by at least one other tool (e.g. Import the FM in Enterprise Architect). This tool will become a tool in the HL7 suite of tools. 68 Final Report HL7 Meeting—Atlanta, USA (May 2013) PROJECT ACTIVITY/ISSUES AT THIS MEETING Michael Van der Zel (The Netherlands) presented an overview of the "EHR Profile Designer Tool”, which is based on the Enterprise Architect product, developed by Sparx Systems in Australia. He recommends using the latest version (version 10) of Enterprise Architecture. The tool is loaded with the content of the EHR-S FM input to the model as XML which was produced from the PHR-S FM spreadsheet developed by Corey Spears and which provides the tool’s instantiation of the EHR-S FM conformance validation rules, formatting rules, and other rules. Once the EHR-S FM is loaded, the tool can be used to generate profiles. He reported that the installation process is simple but care is required as previous installations can be easily deleted during the installation process. During discussion the following issues were raised: • Recording and tracking of changes over time. Helen Stevens explained how spreadsheets could be used to record changes to be fed into the tool when using subsequent versions of the model and/or profiles • Work is expected to result in a June release in according to the current timetable • During phase 2 of the work, consideration should be given to tracking changes in the rules database and profiles. • Whether the PHR-S FM should become just another set of profiles for the EHR-S FM? It was agreed that this is not currently planned but could be considered down the track. In the meantime the two models would be loaded into the tool separately 2.11.2.5 PROJECT: EHR FM R2 GLOSSARY UPDATE PROJECT OBJECTIVE The HL7 Electronic Health Record System Functional Model (EHR-S FM) Glossary is an HL7 reference document that provides a set of definitions and guidelines in order to ensure clarity and consistency in the terms used throughout the functional model. The Glossary includes the definition of important terms used in the expression of EHR systems’ functionalities, and comprises a consensus-based list of Action-Verbs and specific guidelines for constructing conformance criteria (CC). HL7’s EHR WG intends to continually unify the glossaries that support both the EHR and Personal Health Records (PHR) System Functional Models and align them with terms used in other HL7 domains and specialist areas. The need for this has been highlighted by inconsistencies and ambiguities in the EHR-S FM work, mismatches identified by the FIM project between terms used in the functional model and related concepts/terms in the information modelling and enterprise contexts, and some profiling activities. It is expected that Functional Profiles (FP) created within the context of the EHR-S FM will align with and respect this Glossary noting that some context-specific differences may still be required and these will be documented in complementary glossaries. Continuity with previous EHR-S FM versions is provided by including glossary terms that have been deprecated, accompanied by suggestions for preferred replacement terms. Vigorous efforts were made to reduce the ambiguities inherent in the use of human language, to respect the fundamental meaning of words and to avoid domain specific usage of terms. Where definitions of terms are taken from recognized sources, specific references are being included. 69 Final Report HL7 Meeting—Atlanta, USA (May 2013) PROJECT ACTIVITY/ISSUES AT THIS MEETING At the previous HL7 WGM in January, this work was being progressed by a small glossary working party led by John Ritter (US), Mark Brueckl (US) and Mark Janczewski (US). It was understood that the target for completion was some 6 weeks from the end of the WGM (i.e. in late February) and that the resulting harmonised glossary would be included in the next EHR-S FM R2 ballot, which closed on 29 April 2013. This activity was not specifically discussed further at this WGM and appears to have been completed; however, harmonisation with Security WG and RMES may result in some more glossary updates needing to be more broadly harmonised as part of routine harmonisation. It is recommended that this topic be deleted from the list of current projects to be reported at the September 2013 HL7 WGM and Plenary. 2.11.2.6 PROJECT #995: USABILITY GUIDELINES FOR EHR SYSTEMS PROJECT OBJECTIVE This project was originally focussed on providing guidance/criteria on the usability of systems used at the point of care. The motivation for the project was inspired by the guidelines on aircraft cockpit design presented to senior members of HL7 at a recent AMIA conference. The AMIA presentation apparently stressed the importance of human factors and reasonably consistent presentation in minimising the time to respond to crisis situations and avoiding costly errors. Discussion of this topic at the January 2013 WGM had identified that this was potentially a complex area in which a lot of work had already been done and in which there are strong proprietary interests. It was therefore recognised that any work by HL7 in this area needed to be informed, collaborative and build on both previous and concurrent activities, some of which were identified at that WGM. PROJECT ACTIVITY/ISSUES AT THIS MEETING Based on the feedback from the January 2013 WGM, a draft Project Scope Statement (PSS) had been prepared and was supported by the Steering Division on 22 April. It was subsequently forwarded to the TSC, which suggested a number of changes to the originally proposed approach. At this WGM, there was considerable discussion within EHR WG of how this item would be progressed, with the following points being noted: • In their consideration of the proposed project, the first issue raised by the TSC was the extent to which HL7 is limited in its activities to interoperability standards and guidelines. It was noted that the EHR-S FM and PHR-S FM are precedents for standards that go beyond this narrow interpretation. They provide frameworks for assessing systems functionality but they do not breach the long-standing principle that HL7 should avoid attempting to standardise the features of EHR systems. The proposed work on usability should also respect this principle. • The TSC also requested that the background review of existing literature and work (originally put forward as a second step) should be done as a clearly defined first step – and before moving to recruiting a broad range of experts from across many intersecting fields to undertake the subsequent work. • The goal of this project is now focussed on translating existing, well-established usability guidelines and health information management principles into functional criteria within the EHR System Functional Model (EHR-S FM), rather than developing a separate “EHR Usability” specification. 70 Final Report HL7 Meeting—Atlanta, USA (May 2013) • Within EHR WG there is quite a lot of support for integration of this activity with the work on Clinical Quality Information (CQI) by the CIC, which is, in turn, related to US-realm needs for quality measures in MU Phase 3. TSC has indicated concerns about basing a Universal Realm (UV) specification too reliant on US-realm requirements but there is still a strong push to progress the CQI relationship in some way, possibly through parallel development of related functional profiles that would test out the UV-realm extensions to the EHR-S FM. • The next steps identified in the PSS are the development of a usability framework against which the EHR-S FM can be assessed and, finally, the incorporation of relevant functions and criteria into the EHR-S FM, relevant functional profiles and, possibly, the identification of other types of guidelines or specifications to support usability. There was a lot of speculation on how this might be approached, which will become clearer as the project progresses. The EHR WG is setting up a Usability Sub-Group to hold regular conference calls to progress work on this project (in the form it is finally approved by TSC). Participation by Australians with interest and existing expertise in the area would be welcome. 2.11.2.7 PROJECT: #1008: MEANINGFUL USE EHR SYSTEM FUNCTIONAL PROFILE (OF EHR SYSTEM FUNCTIONAL MODEL RELEASE 2) PROJECT OBJECTIVE This project aims to develop a “Meaningful Use” EHR System Functional Profile (FP) by identifying functions/criteria from the EHR-S FM Release 2, pertinent to US Meaningful Use (MU) Stages 1, 2 (and 3, as it develops) and aligning these with the associated ONC 2014 Certification Criteria. The project is being led by Gora Datta and will use the Enterprise Architect (EA) based EHR Tooling to develop the functional profile. Availability of the MU functional profile will provide the potential for EHR Systems to be simultaneously certified against MU1, MU2 and MU3 criteria and related EHR-S FM criteria. PROJECT ACTIVITY/ISSUES AT THIS MEETING This project is just commencing. The proposed project scope statement (PSS) was reviewed and supported by the Structure and Semantic Design Steering Division at this WGM and at the time of writing had been submitted to the TSC for approval. The project responds to the US-ONC suggesting that it might be good to use certification against ISO/HL7 10781 (the EHR-S FM) as a means of providing an added value to US stakeholders, whereby, if vendors' systems achieved certification in the US, those advances might be leveraged in non-US realms. The two project leads, Julie Richards and Gora Datta, invited participation in the project; the team utilizes the EHR Interoperability WG conference call coordinates every 2nd and 4th Tuesday at 1:00 PM US Eastern Time. 71 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.11.3 ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by EHR WG Issue: There is a mismatch between the HL7 and ISO re-ballot requirements in relation to the next round of normative balloting to finalise the ISO/HL7 10781 EHR-S FM R2 standard. The leaders of EHR WG have sought advice and assistance on managing the potential issues through the JIC Chair (Richard Dixon Hughes), the ISO/TC 215 Secretariat (Lisa Spellman), and the ISO/TC 215/W G1 Secretariat (Naomi Ryan). ISO/TC 215 WG 1 Secretariat (Naomi Ryan) Re-ballot of R2 EHR-S FM Richard Dixon Hughes Action: Naomi Ryan (WG1) and Richard Dixon Hughes to consider issues relating to harmonisation of DIS2 ballot of ISO/HL7 10781 with HL7 N3 and assist HL7 EHR WG Co-Chairs and ISO/TC215 secretariat in finalising a joint publication in the most effective manner. EHR WG HL7/N2 and ISO/DIS2 ballot of PHR-S FM Issue: An Australian review and response to these two ballots of the ISO/HL7 PHR-S FM will be required in coming months. This was previously covered by IT-014-09 but now falls under IT-014-13 with its broader responsibility of “Clinical workflow and care systems”. The HL7/N2 ballot through HL7 Australia is likely to close much earlier than the ISO/DIS2 ballot. A consistent approach is desirable. IT-014 Secretariat HL7 Australia Action: IT-014-13 and former members of IT-014-09 to be invited to prepare ballot responses to HL7/N2 and ISO/DIS2 ballots in collaboration with HL7 Australia. EHR WG Proposals for R3 of EHR-S FM Issue: While preparation of Release 3 of the EHR-S FM is still some time away, the preparation of the PSS for this work is already being progressed by Functional Information Model (FIM) Sub-Group, who are recommending a fundamental re-engineering based on incorporation of their current approaches. While this may or may not have merit, the preliminary work on the FIM is strongly U.S.based and there is a risk that the International applicability of the EHR-S FM as an international standard will be prejudiced or lost in this process. It is desirable that this move be closely monitored and, if necessary, addressed at an early stage to ensure that any outcomes are internationally applicable. IT-014 HL7 Australia Australian delegations to HL7 WGMs Action: Future delegations, IT-014 and HL7 Australia monitor the proposals and scope statement for R3 of the EHR-S FM to ensure that future revisions are likely to remain relevant as an international standard. As the HL7 process for approval of Project Scope Statements is an internal function of the TSC without external input, raising early harmonisation of this work through JIC should be considered. 72 Final Report HL7 Meeting—Atlanta, USA (May 2013) Topic Issue/Action/Recommendations for Australia EHR WG Issue: Participation in the work of the new Usability Sub-Group of the EHR WG by Australians with interest and expertise in usability of systems would be welcome. The project is commencing with a literature review and white paper, with a view to identifying relevant usability criteria and incorporating them into the EHR-S FM. Australian and related ISO/TC 215 guideline documents on user interfaces and the application of clinical decision support are among the key references identified to date. Usability Sub-Group Recommended for Action by IT-014 HL7 Australia Action: IT-014 and HL7 Australia to circulate information on the HL7 EHR Usability Project among relevant experts with an invitation for them to participate in the activities of the Usability Sub-Group of the EHR WG. 73 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.12 ELECTRONIC SERVICES 2.12.1 PROGRESS AT THIS MEETING Progress at the Atlanta WGM for this WG was substantively impacted by the absence of three of the four Co-Chairs who had been managing a number of the key projects of the WG. The WG had planned to support the virtual attendance of Ken McCaslin (an ES Co-Chair) using a Google Hangout. This required the supply and setup of equipment with video and microphone that was projected using the Audio Visual equipment in the meeting room. While the equipment functioned correctly and 3 WG members were able to be linked into the virtual meeting that was projected on the meeting room screen, Ken was not able to join the session because of restrictions in his corporate firewall. The ES WG has been trialling technologies such as Google Hangout to support virtual attendance at HL7 meetings that provide a more integrated approach to participation than one of more of teleconference and WebEX connections. HL7 International policy is to disallow virtual attendance, though it is not uncommon for Co-Chairs who are not able to attend a WGM to “dial in” over a phone connection. At this WGM, the level of sophistication of such attendance ranged from a mobile phone passed around the room, to the ES WGs attempt to have shared video, desktop and remote presentation capability. Given the current common but somewhat illicit participation of HL7 Co-Chairs, the need for Co-Chair participation by all Work Groups, and for HL7 staff members in Technical and Support Services Work Groups, allowing and more so supporting virtual attendance for Co-Chairs and HL7 Staff at a WGM ought to be facilitated. Nat Wong led this dialogue and has been tasked with liaising with HL7 International to table the request. At the request of Nat Wong, Dave Hamill presented an update on the provision of a HL7 Help Desk. At the Phoenix WGM, this Help Desk had been provisionally focused on the supporting HL7 Tools. The scope in Atlanta for this help desk appears to suggest a much broader role including the participation of onsite HL7 support. Dave indicate that HL7 International had begun exploring different Help Desk systems, however as the HL7 Board had yet to confirm any particular Help Desk function, Help Desk software review and selection remained at a preliminary investigative stage. Dave Hamill was also able to provide an update on changes to the HL7 Membership software system. Dave indicated that Mike Kingery (Director of Technical Services) and Diana Stephens (Director of Membership Services) have been working on changes to the GoMembers membership software used by HL7 International. While the HL7 Board was yet to confirm the exact nature of the new membership tiers, it was expected that the software changes that had been implemented would cover the scope of membership tiers that had been tabled. These changes are expected to take effect somewhere between July and September, however it is unlikely that these changes would impact on the day to day use of the HL7 website. During the T3SD Steering Division meeting, in response to a discussion with John Ritter and Diego Kaminker in their roles on the International Mentoring Committee, Nat Wong mentioned the work that the HL7 Australia ES committee had been discussing on the concept of an “Affiliate in a Box”. While this project was still in its formative stage and focused strictly on the provision of a reusable Infrastructure platform, interest was expressed in keeping up to date with this work by the other Steering Division participants. 74 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.12.2 CURRENT PROJECTS The following projects are reported on in this section: • Project #880: HL7 Website Enhancements • Project #877: Develop HL7 Ballot Site Education Tutorial 2.12.2.1 PROJECT #880: HL7 WEBSITE ENHANCEMENTS PROJECT OBJECTIVE The goal of this project is to enhance the existing HL7 website with new and extended functionality. The Project is preceded by Projects #786, #785 and #674 that also dealt with enhancements to the website. Each of these projects embodies incremental extensions both to the internal intranet resources used by HL7 HQ and the external/public Internet. PROJECT ACTIVITY/ISSUES AT THIS MEETING No progress was made on this project at this WGM. 2.12.2.2 PROJECT #877: DEVELOP HL7 BALLOT SITE EDUCATION TUTORIAL PROJECT OBJECTIVE The intent of this project is to develop a tutorial on how to use the HL7 Ballot site. This tutorial will be developed in English. PROJECT ACTIVITY/ISSUES AT THIS MEETING Don Lloyd presented a brief progress update on the progression of the Ballot site tutorial material, indicating that the tutorial content had been developed, but had yet to be recorded. In the absence of the majority of the key players in this project, further discussion of this project was deferred to a later meeting. 2.12.3 ACTIONS FOR AUSTRALIA Topic Issue / Action / Recommendations for Australia Recommended for Action by Electronic Services/ T3SD Issue: Work in Australia on update of its electronic services may be complementary to other HL7 work. HL7 Australia Action: Keep the Technical and Support Services Steering Division updated with the progression of the “Affiliate in a Box” concept being developed by HL7 Australia. 75 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.13 2.13.1 HEALTH CARE DEVICES PROGRESS AT THIS MEETING The meeting in Atlanta was a joint meeting of IEEE EMBS 11073 and HL7 Health Care Devices (DEV) WG. 2.13.1.1 REVIEW OF VENTILATOR NOMENCLATURE ISSUES 11073 Nomenclature to be revised as current standard for measurements is 10 years old for ventilator or anaesthesia machine. The changes remove the need to pre-coordinate the different measurements into the terminology as units of measures. Need to assign identifiers that include default point of measurement, inspiration and expiration, i.e. link to breathing cycle. Discussion on acronyms and their reflection of what is being measured – and what it is measuring semantic refinements needed after point of flow, concentration, gas and phase (Inspir, Exspir) = phase of ventilator, patient or circuit. Suggestion for category for airway measure consolidated (MDC_CONC_AWAY, MDC_VENT_AWAY). Whilst clear on diagram as you add more concepts it becomes less clear in practice what is being measured at what point. It was demonstrated that there are many synonyms in current standard. The work needs to devise improved guidelines and possibly consider deprecating some of these terms. 2.13.2 ACTIONS FOR AUSTRLIA Topic Issue / Action / Recommendations for Australia Recommended for Action by Health Care Devices: Ventilator Nomenclature Issue: Standardisation of measurement points and variables. Standards Australia (HE-003) Action: Advise Standards Australia Health Care Devices mirror group HE-003 of revisions and canvas for potential members or manufacturers to engage in this work. 76 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.14 HL7 ACTIVITIES WITH OTHER SDOS 2.14.1 PROGRESS AT THIS MEETING The agenda for the Sunday Q4 session of this group identified some 30 potential liaison reports that could be covered. Of these, reporting was largely by exception and by those present and included the following: • DICOM (Harry Solomon). Development of web‐based access mechanisms is continuing, including working with FHIR. DICOM WG‐27 was holding a joint meeting with the web services group at this WGM. They are also having vocabulary cleanup in the dental realm. • IHTSDO (Russ Hamm) IHTSDO Workbench. The Vocabulary and Tooling WGs are continuing with activities to trial and integrate the IHTSDO Workbench into HL7 Vocabulary harmonisation and, ultimately, management of al content. He anticipated that this would need to be a funded project effort. U.S. SNOMED CT® Content Request System (USCRS). The IHTSDO has begun to use the USCRS that was produced by the US-NLM. The USCRS provides users with the ability to request basic changes to SNOMED CT via a web interface and allows for those requests to be analysed and triaged by the vocabulary maintenance team. A proposed Vocab WG project for HL7 to trial USCRS has the potential to streamline HL7 vocabulary harmonization and would serve to align HL7 vocabulary tooling with that of the IHTSDO and the NLM. John Quinn (CTO) noted that a core of some 20 HL7 vocabulary/terminology experts also regularly attend IHTSDO. Some including Kaiser Permanente also participates in CIMI, supporting communication between these groups and efforts to build UML micro‐models. Jim Case supplemented these reports. He noted that all SNOMED CT editing has now moved from CAP to be done in-house at IHTSDO; the web-based USCRS is now used as the request submission system. Known as “SIRS”, the IHTSDO version of USCRS will only accept requests from authorised release centres, which will shortly include the HL7 Terminology Authority (HTA) [see notes on HTA in the Board report]. IHTSDO is hiring three editors and is also introducing a one-year apprenticeship-style program to train consultant terminologists able to action large and small ‐ terminology changes and model/design national terminology releases. There is also the SNOMED implementation advisors group, another channel for communication with IHTSDO. There will be an implementation showcase at the IHTSDO conference at Washington DC in October. Ann Wrightson advised of the UK Terminology Centres implementation forum and their published webinars that are publicly available. • ISO/TC 215 (John Quinn). HL7 would like to achieve greater coordination among the 20 or so HL7 experts that attend ISO/TC 215 meetings and, also, have HL7 officially receive communications for all ISO/TC 215 WGs. He also mentioned HL7 commitment to SKMT and the issues associated with managing HL7 updates [see CTO report under HL7 Board for more information]. Richard Dixon Hughes also reported on ISO/TC 215 noting that changes to WG structure are complete and the new business plan has been approved in principle. A proposed re-statement of TC 215 scope 77 Final Report HL7 Meeting—Atlanta, USA (May 2013) has raised some issues with IEC/TC 62 (Medical Devices) and ISO/TC 121 and is being worked through with assistance from Todd Cooper and Sherman Eagles and others well known in HL7. The next ISO/TC 215 meeting will be in Sydney on 21-25 October and will be followed by IHIC 2013 early the following week. All HL7-ers are welcome. Ted Klein and Woody Beeler highlighted a project that they are leading in ISO/TC 215 for using core principles for binding of terminology with ISO 21090 data types. It promotes using HL7 normative mechanisms for terminology binding to data types. • JIC (Richard Dixon Hughes) An update was provided on the April JIC meetings, held in conjunction with the ISO/TC 215 meeting in Mexico City. In addition to thanking John Quinn for HL7’s contribution to JIC, Richard spoke to a brief presentation, available at: http://gforge.hl7.org/gf/download/docmanfileversion/7324/10423/JICISO-TC215Atlanta.pptx (accessed 16 June 2013). Points highlighted in speaking to the presentation included: - progress on the strategy for engagement with LMIC, building on the recommendations of the ISO/TC 215 Public Health Taskforce and opportunities to progress collaboration through professional societies (notably IMIA), global agencies such as WHO and ITU and funder/donors. - IHE’s recently joining of JIC and the benefits in terms of strengthening the focus on implementation for JIC joint projects. - The importance of other SDOs, including HL7, becoming involved in balloting of cornerstone standards on the JIC list of joint projects. ISO 13940 System of concepts for continuity of care (ContSys) has recently been out for DIS ballot and provides a framework of concepts, terms and definitions that should be underpinning the vocabulary used across the JIC members and also the terms and definitions preferred in SKMT. It is vital that HL7 is involved in the development of these artefacts and uses them as shared foundations. - Formatting problems have been overcome and EHR‐S FM, PHR-S FM are being balloted simultaneously in HL7 and ISO/TC 215/WG1. • OASIS (Elysa Jones) It was reported that OASIS had developed an MOU with HL7 for Tracking of Emergency Patients (TEP) standards, initially addressing TEP transform to HL7 V2 or V3 messages to be balloted in the next cycle. OASIS will also be bringing in Hospital Availability or HAD into HL7 as a project. • Regenstrief/LOINC (Ted Klein) Ted mentioned his involvement in the February LOINC clinical conference and proposed updates in LOINC licensing arrangements. He provided a brief presentation, available at: http://gforge.hl7.org/gf/download/docmanfileversion/7310/10400/LOINCliaisonreportMay2013.pptx (accessed 16 June 2013). Jim Case reported that final the signed version for LOINC/IHTSDO Collaborative agreement on use of LOINC with SNOMED was imminent. From a practical viewpoint, vital sign ontology and document ontology are primary areas of initial focus for alignment. Planning session (Q3 Monday). After a brief discussion (which was attended by Richard Dixon Hughes en route to another session), the group dispersed with a resolution to set up a conference call in several weeks 78 Final Report HL7 Meeting—Atlanta, USA (May 2013) to review future plans for TSC's Activities with other SDOs communication. There is some concern within HL7/TSC that the traditional role of the Sunday session is an additional overhead for TSC and attendance is down as it is competing with more and more other activities (such as free FHIR tutorials). It was indicated that the Australian delegation would prefer that this Sunday session be retained as it one of the few opportunities for TSC to support HL7’s commitment to joint activity. 2.14.2 ACTIONS FOR AUSTRLIA There are no actions for Australia specifically arising from this activity, although some of the matters reported here are the subject of actions raised elsewhere. 79 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.15 HL7 ARCHITECTURE PROGRAM AND SAIF 2.15.1 PROGRESS AT THIS MEETING Each project from the SAIF architecture program provided a brief overview of their status. Some projects have reported no or insignificant progress due to lack of resources, e.g. SAIF Implementation Guide, SAIF Artifact Definition (led by MnM) and Composite Order, so most of the meeting was focused on describing progress from two projects, namely: • Project # 587 - Lab Order Template (OO) and • Project # 863 - Cross-Paradigm Interoperability Implementation Guide for Immunizations project (“X Paradigm”) (SOA) Note that Project # 863 - Cross-Paradigm Interoperability Implementation Guide for Immunizations project (“X Paradigm”) (SOA) is discussed in the SOA WG description section of this report. Note also that the items recommended for Actions for Australia identified at the Phoenix WG meeting are still open and carried into this report. They were not addressed due to the insignificant progress in the related projects since the Phoenix WG meeting. 2.15.2 CURRENT PROJECTS The following project is discussed in this section: • Project: SAIF Artifact Definition • Project: Project # 587 - Lab Order Template (OO) 2.15.2.1 PROJECT: SAIF ARTIFACT DEFINITION PROJECT OBJECTIVE The goal of the project is to identify and define the artefacts to be created and used as part of HL7 standards development work. The wiki page provides a means for formally defining all of the artefact types that will be developed, maintained and published by HL7 WGs under the SAIF methodology. This content will form a significant portion of HL7's SAIF IG. The artefact definitions attempt to identify the purpose for a given artefact, the reasons for its existence as well as the various constraints on when and how it is used and the content it may contain. Further information can be found at: http://wiki.hl7.org/index.php?title=Category:SAIF_Artifact_Definition [Accessed 23 January, 2013] PROJECT ACTIVITY/ISSUES AT THIS MEETING This and the following project were discussed at length and are summarised below. Of interest for SAIF Architecture program is that the X-paradigm and Lab Order template projects took different modelling approaches, in that the X-paradigm is looking breadth wise, rather than diving down the specification stack. These two groups met to discuss early in the effort, and decided that it was worthwhile for each to take separate paths, and then come together to discuss lessons learned nearer the end, so we could compare and contrast artefacts for specific uses. 80 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.15.2.2 PROJECT: PROJECT #587: LAB ORDER TEMPLATE PROJECT OBJECTIVE To develop specifications and models for communicating orders for laboratory (specimen-based) testing using the new, alpha, just developed, Service Aware Architecture Framework (SAIF). SAIF specification is at the conceptual or canonical description level; currently there is no v3 SAIF Implementation Guide for HL7. Therefore, the work in this laboratory conceptual specification is best-guess and alpha-level thinking. The project team believe the current artefacts thoroughly describe the interoperability space for laboratory order; however, those exact artefacts are not necessarily the artefacts that will eventually be contained and defined by the SAIF IG and are presented as draft and present for your consideration. Further information can be found at: http://wiki.hl7.org/index.php?title=Lab_Order_Template [Accessed 19 May, 2013] PROJECT ACTIVITY/ISSUES AT THIS MEETING Lorraine Constable, Modelling, SOA Facilitator reported some progress in the last quarter. The majority of progress relates to the expression of requirements using the Sparx EA tool and specification of the Conceptual Roles and Conceptual Information Model. There was also initial work on requirements traceability. The next steps are to work on the logical specification, taking into account the Lab Order RMIMs, and to further focus on traceability through the SAIF matrix. 2.15.3 ACTIONS FOR AUSTRALIA As per previous meeting recommendations Topic Issue / Action / Recommendations for Australia Recommended for Action by HL7 Architecture Program and SAIF: Issue: It is not clear at present how Product Line/Product Family concepts from the ARB BAM project are related to the SAIF Architecture Program. HL7 Australia SAIF Artifact Definition Action: Contribute to and influence product Line/Product Family developments while linking it with the experience and deliverables from the SAIF Artifact Definition project. 81 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.16 INTERNATIONAL COUNCIL—GENERAL SESSION The following 14 affiliates were represented at the general session by their Chair or another nominee from their affiliate: HL7 Argentina, HL7 Australia, HL7 Brazil, HL7 Canada, HL7 France, HL7 Germany, HL7 India, HL7 Italy, HL7 Japan, HL7 The Netherlands, HL7 Norway, HL7 Puerto Rico, HL7 Switzerland, and HL7 UK. A representative of the USA membership also attended. The following 5 affiliates were represented by a proxy from another affiliate: HL7 Colombia, HL7 Czech Republic, HL7 Korea, HL7 New Zealand, and HL7 Russia. 2.16.1 PROGRESS AT THIS MEETING Matters addressed in general session included: • $3,500 USD had been approved for HL7 UK to support a meeting of European affiliate Chairs in Ireland in eHealth week, May 2013. • HL7 Education WG Report. The following were particularly noted: - Increased activity and focus since the appointment of Dr Susan Chaplock as Director of Education. - Continued delivery of HL7 e-learning courses (HL7 Fundamentals) - Development of Portuguese- and Japanese-language editions of the e-Learning program - Development of additional e-Learning material on FHIR, authored by David Hay (NZ) - New hands-on workshops at the educational summits - Electronic outreach - new MU webinar series; new skill building for certification webinars; new education portal (due June 2013) • Preparing for electronic certification testing in July 2013 Membership Committee. This committee was formed by the HL7 Board. Its role, membership and activities are reported in more detail in the section of this report on the HL7 Board. Philip Scott and Diego Kaminker were confirmed as International Council representatives on the Membership Committee. There was some discussion regarding the progress and scope of this committee and the Council’s desire to provide additional support to Philip and Diego and representation from the international community. It was agreed that Diego and Philip would discuss with Grant Wood (Chair of the Membership Committee) the addition of Bernd Blobel and Melva Peters to the Membership Committee. ELECTIONS Helen Stevens was elected as International Council’s to the HL7 Nominations Committees. Confirm International Council representatives on Membership Committee – Philip Scott, Diego Kaminker, and Melva Peters and Bernd Blobel newly elected AIR MILES COLLECTION PROPOSAL This initiative is to provide expertise to new affiliates at a lower cost. It has been suggested that air miles that are about to expire may be donated to HL7 for members to be able to subsidize travel to access 82 Final Report HL7 Meeting—Atlanta, USA (May 2013) expertise. This would include international Council members from less well-off affiliates and LMICs to WGMs. This would need robust governance rules about the program. Investigation is underway on a program for this. FHIR UPDATE A brief introduction to FHIR from an international perspective was given by Lloyd Mckenzie, covering key features and references to further information for follow-up via the following channels: • Read the specifications and other commentary at: hl7.org/fhir • Follow #FHIR on Twitter • Shape the specification by making comments online (using the wiki linked to hl7.org/fhir) • Attend FHIR sessions at WGMs • Try implementing it and/or come to a FHIR Connectathon • Email contact: lloyd@lmckenzie.com The focus for FHIR continues to be on implement-ability and international specifications (AU, Canada and Netherlands). The management group emphasised that FHIR is licensed under a different agreement to other HL7 products and that no share back requirement for developers using FHIR is currently in place. FHIR was discussed as another key aspect for membership going forward and an opportunity for strategic marketing by HL7. EDUCATION UPDATE Education was emphasised as one of the strongest earners for HL7. In context of the Chair report education was also referred to as an important aspect for membership value. The importance of member access to development of HL7 knowledge through training, certification and access to materials was highlighted. Some significant deliverables include: (1) Education portal to be available in approximately June this year via the website. This will have links to registration and program information to improve access to curricular. (2) Development of E learning for FHIR has progressed to the point that it is about to be launched. This has been developed with David Hay (NZ). (3) Electronic outreach will be ongoing in the form of power points available as webinars on the website. (4) Preparation for electronic certification is continuing. HL7 INTERNATIONAL BOARD There was an intense discussion over the composition of HL7 International Board. Many affiliates are concerned over the distinction between the 7 “Directors at Large” elected/appointed from the HL7 International direct membership, who are predominantly from the US, and the 2 “Affiliate Directors” elected from the Affiliate membership. Under the constitution these numbers can be up to 8 and 4 respectively. It was proposed that it is preferred to have a better geopolitical balance of these directors. A potential motion was put forward: “Motion to express the will of the Council that a phased proposal be developed to remove the distinction between directors at large and affiliate directors and to have equitable voting rights for HL7 International and affiliate members as well as a balanced geographical representation structure at the HL7 Board.” 83 Final Report HL7 Meeting—Atlanta, USA (May 2013) Australia’s position is that this is insufficiently discussed and formulated at the present time. This will be further discussed at the meeting later in the week. AFFILIATE VALUE PROPOSITION TASKFORCE There has not been any progress since the last meeting, however an overview of how this has been approached in the UK of the possibilities by Philip Scott. The UK analysed the spread sheet of HL7 benefits, identified what offered to UK community members, and the themes broadly were education, implementation support, marketing and strategic guidance. This includes localisations, help desk, user groups, vendor showcase, products and service directories, educational discounts, and access to UK profiling and tooling. What was identified was to be of value would be: • Support HL7 implementations • Provide marketing opportunities for vendors • Provide education support for next generation implementers • Validate products and people • Access to UK products and profiles. A draft business plan has been done for each of these areas to see how this would work. The discussion and progression of this is on-going. CONFERENCES Richard Dixon Hughes gave an update on IHIC 2013 in Sydney on 28 and 29 October 2013. In addition there will be a parallel FHIR Connectathon. The EFMI STC (special topics conference) on Data and Knowledge for Medical Decision Support had taken place in Prague on 17-19 April (brief report given by Bernd Blobel). 2.16.2 2.16.2.1 CHIEF TECHNOLOGY OFFICER (CTO) REPORT SKMT Four years ago Heather Grain approached HL7 (Ed Hammond, John Quinn and others) for HL7 support and contribution to an ISO TC 215 Health informatics "Glossary project" called SKMT (Standards Knowledge Management Tool). HL7 International contributed a number of glossaries and acronym lists that HL7 and its members had created over the life of HL7 to this project. It was requested that members of HL7 start using SKMT and provide feedback on its use. The SKMT website can be found at www.skmtglossary.org. Registration is required but has no cost. All registered users currently have edit rights that are constrained only by the membership organization defined during user registration. There are otherwise no bounds on edits, so John cautioned people that rollback/undo functionality is not yet well defined. It was requested that anyone making changes should contact the Project committee, and to otherwise refrain from making changes, or if making changes, to only change definitions for which they have some level of responsibility within HL7. Currently HL7 entries of terms belong to a single 'document' (or node). 84 Final Report HL7 Meeting—Atlanta, USA (May 2013) RIM IN OWL 2.16.2.2 At the Phoenix WGM HL7 kicked off a tooling project based on the idea from Lloyd McKenzie that the RIM could be expressed in OWL. This approach proposed the creation of an XML expression of the RIM that could be imported into an off the shelf OWL tool. In March HL7 International received a 300-page XML representation of the RIM from Lloyd. Using Protégé (an open source ontology editor and knowledge base framework), this was able to be loaded as an XML file. The utilities used to create the XML file will be made available before the next meeting. In the interim, the current RIM XML file would be made available on the HL7 web page. This XML file is one revision behind the current version. The utilities used to convert the MIF need to be made more robust. The project was presented this project to CIMI, however the response was less warm. Berndt Blobel indicated that the relationship with CIMI was complicated. 2.16.2.3 MORE TOOLING The Tooling Strategic plan was unanimously approved by the HL7 Board in a Teleconference after the Phoenix WGM. The Tooling WG will embark on a Tooling Tactical plan prioritised by the decisions of the membership and policy committees and the HL7 Board as soon as those decisions are made. The Tooling Tactical plan needs to be aligned with the new membership structure and is likely to preoccupy discussion on Tuesday. The Tactical plan cannot be progressed until the membership structure has been worked out, as the two are tightly coupled. 2.16.3 TECHNICAL STEERING COMMITTEE (TSC) REPORT The TSC report was presented by Austin Kreisler. The WG Health measures/metrics are being extended to the mid-tier governance groups including FHIR. This includes the TSC and Steering Divisions. The International Council has been asked if they would also like to be measured. The metrics are still being determined, but a draft copy of the expected metrics is available on the TSC website. Melva Peters (HL7 Canada) has taken on the tasks to clean up the metrics for the Steering Divisions. TSC elections are occurring this summer. TSC is forming a nomination committee. The TSC has a number of strategic projects in progress. These include the following: • HL7 Business Architecture Modeling (BAM) project: The intent of this project is to develop a business architecture that incorporates Product lines and Families. This project is already underway. The first pilot is FHIR and is an 'experiment' using the SAIF governance model. The second pilot is a CDA IG family. This is taking an existing standard as a product family. This is considered a test. The HL7 Product line Architecture program is tasked with doing the work of standing up the projects in relation to the model. • SAIF Project: The task of rolling out SAIF across HL7 International is currently stalled. A permanent committee has been established to do risk assessment and define governance points for the TSC. The committee is analysing source documents such as the GOM, trying to surface the risks for which the mitigations were originally designed. This is a somewhat interesting way to recover those risks in the absence of any other organisational knowledge. ANSI’s audit of HL7 international balloting practices has resulted in a change that will impact Work Groups. There is now a 1 year deadline to complete reconciliation and publish. If further time is required then TSC 85 Final Report HL7 Meeting—Atlanta, USA (May 2013) approval must be sought, as the TSC needs to notify ANSI about the extension. There will be some leniency in the publication dates as there are some clear cases in which exceptions will be required. The ANSI audit also suggested that HL7 International’s Governance and Operations be separated from the ANSI compliance process. Otherwise, all HL7 internal processes are evaluated. This will effectively mean the creation of two Operations manuals, so ANSI will stop grading HL7 on how it deals with its own internal processes. 2.16.4 REGIONAL REPORTS HL7 EUROPE 2.16.4.1 Currently Europe HL7 has 19 Affiliates. Current and/or planned HL7 Europe involvement in the following European projects and activities were noted: • epSOS: Second phase approved and funding provided. Increasing reliance on CDA provides opportunities for greater SDO collaboration. • Semantic HealthNet: (HL7 project lead: Charlie McCay). Annual review in February commended HL7 work. A further update was expected in April. • eHealth Governance Initiative: eID workshop February 10-11 in Brussels, focused on the specific authentication and identification requirements of eHealth. • ANTILOPE project: (HL7 project lead: Catherine Chronaki) Initiated in February. Focus will be testing; its first meeting including SDO members will be in Dublin on May 16. • HL7 workshop at WoHIT on May 13 prior to Ministers meeting as part of the eHealth Europe week in Dublin. • The next HL7 Europe newsletter is planned for production in time for the eHealth Europe week in Dublin. 2.16.4.2 HL7 ASIA Following the formation of HL7 Asia with funding from respective ministries of health in China, Japan, Korea, Taiwan, Hong Kong and Singapore and the election of Ken Toyoda (Japan) as its first Chair, HL7 is planning engagement through: • HL7 Asia Meeting. This will be the inaugural meeting of HL7 Asia and is expected to attract some 200 delegates. It is planned to take place in Tokyo on 18-19 July 2013. • HL7 Asia is also collaborating with the organisers of the following upcoming events, with a view to participation and promotion of HL7 Asia: - HIMSS APAC Greater China eHealth Forum being held in Hong Kong from 31 July to 2 August 2013. - HIMSS APAC Digital Healthcare Week (Asian Ministry Conference). Singapore, 21-24 October 2013. 2.16.5 HL7 AROUND THE WORLD A synopsis of developments from reports of international affiliates around the world is provided in Section 4 below. 86 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.17 INTERNATIONAL COUNCIL—AFFILIATE CHAIRS SESSION 2.17.1 PROGRESS AT THIS MEETING The affiliate chairs session at working group meetings is the forum in which the International Council (IC) makes most of its major decisions. The following 14 affiliates were represented at the general session by their Chair or another nominee from their affiliate: HL7 Argentina, HL7 Australia, HL7 Brazil, HL7 Canada, HL7 France, HL7 Germany, HL7 India, HL7 Italy, HL7 Japan, HL7 The Netherlands, HL7 New Zealand, HL7 Puerto Rico, HL7 Switzerland, and HL7 UK. A voting representative of the US membership also attended. The following 9 affiliates were represented by a proxy from another affiliate: HL7 Austria, HL7 Colombia, HL7 Czech Republic, HL7 Luxembourg, HL7 Korea, HL7 Norway, HL7 Russia, HL7 Spain and HL7 Uruguay. With 23 out of 35 affiliates represented, a quorum of the International Council was present. Matters addressed by the affiliate Chairs included: • HQ Liaison and Financial Report (Philip Scott). - The IC budget allocation for 2013 is now fully committed and includes $5,000 already approved for HL7 Australia toward the cost of running IHIC 2013 in Sydney in October. - Requests for the 2014 budget should be submitted at least 35 days in advance of the September HL7 WGM so that they can be distributed and voted upon at the September meeting. - The budgeted allocation of $3,500 to HL7 Taiwan was approved to support funding speakers from Asia (Japan, Korea, and China) for the Asia-Pacific HL7 Conference in October 2013. • Policy Advisory Committee (PAC) membership. Hans Buitendijk (Chair of the PAC) presented an overview of PAC activities and plans. The following points were particularly noted during his presentation and subsequent discussion. The PAC is a Committee appointed by the HL7 International Board and will now be working closely with Ticia Gerber as the newly appointed Director of Global Partnerships and Policy in identifying opportunities for HL7 policy input to government and industry bodies around the world and the exploitation of opportunities for HL7 to partner in projects of global significance. The current members of the PAC are: Keith Boone, Bill Braithwaite, Hans Buitendijk (Chair), Kathleen Connor, Bob Dolin (Executive Committee liaison), Richard Dixon Hughes, Jamie Ferguson, Dennis Giokas, Stan Huff and Ticia Gerber (staff). One of the key PAC roles is collating, drafting and editing white papers, drawing on the expertise of the wider HL7 community. For example, in the US realm, it prepared a response to the recent ONC/CMS regulatory RFI on Interoperability; it is working with CQI WG on a response to the US NPRM on the Inpatient Prospective Payment System (IPPS) in relation to quality measures; and work is also taking place in relation to the roll out of Blue Button. In Canada, US and EU developments in the area of privacy and security of health information are being tracked with further inputs expected to be required. [Bernd Blobel noted an upcoming European by invitation only meeting on next steps in privacy]. Globally, the PAC is tracking activities of WHO and ITU in the promulgation and standardization of eHealth. 87 Final Report HL7 Meeting—Atlanta, USA (May 2013) There is work currently underway on developing better relationships with eHealth initiatives in Latin America and a high-level executive meeting with the Pan American Health Organization (PAHO) is being planned for June to explore roles that HL7 International might play. The PAC has concluded greater regional representation among its members could assist it in identifying other similar opportunities and it is exploring the potential for representatives from South America, Europe and Asia to join the PAC. A question was raised on how this might relate to the European and Asia offices and their roles. The IC considered that it would be ideal if regional representatives are engaged in the HL7 regional groups, where they exist. Contacts for further discussion include Catherine Chronaki, SecretaryGeneral of the HL7 Foundation (HL7 Europe), and the Chair of HL7 Asia (currently Michio Kimura). The IC resolved to request the appointment of regional representatives to the Policy Advisory Committee - initially for South America, Europe and Asia. Hans Buitendijk will take this up with the HL7 administration and HL7 Board and report back to the IC at the HL7 WGM in September. [Note: there is also a brief report on PAC activities at this WGM in section 2.25 of this report]. • International Working Group Meetings Earlier in the meeting, the HL7 Board had resolved that the May 2015 WGM would be an “international” meeting to be staged by the European affiliates in Paris (at the location originally proposed by HL7 France.) There was a lot of discussion within IC about the structure and organisation of the Paris meeting, including the possibility of having a European “plenary” on the Monday morning and “policy tracks”. There was also discussion about the more general problems of getting suitable venues, prices and engagement for HL7 WGMs held outside North America. There were two main outcomes: - The IC resolved to establish a steering group for the May 2015 Paris WGM comprising: Philip Scott (Chair), Helen Stevens (HL7 Board representative), Nicolas Canu (HL7 France), Lillian Bigham, Mark McDougall and Ticia Gerber (HL7 HQ), Beat Heggli (HL7 Switzerland), Kai Heitmann (HL7 Germany), Tom de Jong (HL7 The Netherland), and Carlos Gallego Perez (HL7 Spain). The Steering Group’s responsibility is to focus on a plenary program, Tuesday policy track, securing European participation, securing additional sponsorship, additional educational opportunities and social events. It was confirmed that that there will be sub-committees/groups brought in to do a lot of the organizing, there is no expectation of financial commitment from European affiliates other than HL7 France, HL7 HQ would develop a meeting schedule, HL7 France will establish a local team of volunteers to manage their end of the event. - Given the difficulty of securing appropriate venues and the desirability of holding regular international meetings, the IC resolved to reactivate the existing “International WGM Assessment Team” – Kai Heitmann, Lillian Bigham and Melva Peters and request that they report back to the IC at the HL7 WGM in September on suggested locations and dates for international WGMs beyond 2015. 88 Final Report HL7 Meeting—Atlanta, USA (May 2013) • Special Interest Topic – Political realities in New Zealand by David Hay, Chair of HL7 New Zealand. David’s’ presentation covered the following points: - The funding model involves a mix of public and private service provision, universal health insurance and accident compensation commission funding. - Overall health IT policy is managed by the “IT Health Board” a subcommittee of the National Health Board which, in turn, advises the Government on the planning and funding of the New Zealand public health services. - The IT Health Board provides leadership in the implementation and use of information systems across the health and disability sector and is charged with ensuring that health sector policy is supported by appropriate health information and IT solutions. It has membership representative of a broad range of interests and its remit includes funding of Health IT. HL7 NZ has a seat on the peak IT Health Board. - Health information standards fall under the Health Information Standards Office (HISO). - New Zealand has a health IT strategy that is focusing on computerisation of primary health and community care and the secondary/Tertiary acute care sectors in the initial stages, specifically in relation to referral, discharge summary (both supporting the continuity of care), electronic prescribing, medications reconciliation, GP to GP communication and national specialty systems. - The creation of shared clinical data repositories deriving information from these primary sources will ultimately support shared care, tracking patient visits, e-events, care plans and clinical decision support. - The achievement of this vision is supported by a health interoperability architecture (2011) comprising building blocks (regional and national repositories linked via IHE/XDS), clinical content models (local CCR/archetypes) and document exchange formats (HL7 CDA). - At present, there is national infrastructure for identification, some national, regional and private repositories and widespread use of HL7 V2 messaging, with some CDA documents. Terminological resources in use include Read codes, ICD, LOINC, and SNOMED. - There is a considerable gap to be bridged when the future vision is stacked up against the current state of shared regional/national services, standards, primary and community care systems and hospital systems, most of which are dominated by legacy products and approaches. - David Hay perceives a strong role for FHIR in helping to integrate these disparate resources and HL7 NZ is strongly encouraging the potential of this technology. In concluding, he summarized the particular opportunities and challenges facing HL7 NZ: - The size and location of New Zealand creates challenges in participating in HL7 activities internationally. - We have our own internal challenges – political, funding, human resource - that are doubtless shared by many others. - Excellent relationship with HL7 Australia. - IP Changes had greater impact than expected – though not insurmountable. Support the concept. - Current HL7 emphasis on on-line training/certification is very important to NZ. 89 Final Report HL7 Meeting—Atlanta, USA (May 2013) • International Council Evaluation Metrics. The TSC had invited the international Council to consider developing performance metrics to measure its effectiveness, in line with other governance committees within HL7. This was debated at some length within IC with some affiliates (including Australia) being in favour and others being opposed to a variety of reasons. At the conclusion of the discussion of the motion “that International Council agrees in principle that there should be performance metrics for the Council, the details of which need to be agreed to by the Council” was defeated – with 9 in favour, 12 against and 2 abstaining. • HL7 Board Report. Some relatively minor issues concerning the Membership Committee and the financial outcome for 2013 which was somewhat better than expected were the main points noted. • Proposals to “Internationalize” the HL7 Board. This discussion continued the points which had been raised by Kai Heitmann at the general sessions and commenced with a more detailed presentation of the following concepts: - Removing or reducing the distinction between “directors at large” (some of which are elected and some directly appointed) and the “affiliate directors”; - A proposal for “balanced” geographical representation on the HL7 Board. To be achieved by appointing the Chairs of HL7 Europe and HL7 Asia to the Board (to increase the number of affiliate address directors to the fore currently allowed in the GOM); and - Giving members of affiliates and members of HL7 International equal rights to vote for the Board of HL7 International (the long-standing one member one vote issue). The European affiliates are reported by some of their advocates to be strongly behind these proposals because they continually face push back from the European establishment on the grounds that that HL7 International is a US-dominated organisation. The International Membership and Affiliate Taskforce (IMATF) final report also proposed that some of these changes be considered but that report is not being actively considered at present because of the pressures brought about by the decision to license HL7 standards and other selected IP free of charge. Consideration of this report is understood to be on the longer-term agenda of the Membership Committee. HL7 Australia is in a minority. It understands the issue and concerns but does not see how the proposals are equitable to those who pay considerably more to belong to HL7 International; nor or are they favourable to all affiliates or consistent in stripping away special identification of “affiliate” directors but then replacing them with directors from identified regions. The final resolutions represent the minimum common ground, namely: - Request to the board to change HL7 website and other communications so that all affiliate directors and directors at large are listed as “Directors”. It was understood that this would not require any changes to the voting process or the Governance and Operations Manual (GOM). - Request that the Board activate the additional 2 Affiliate Directors for the 2013 election cycle. (Australia, UK and US abstained). - Further discussion regarding the Internationalization of the HL7 Board would be tabled until the September WGM. 90 Final Report HL7 Meeting—Atlanta, USA (May 2013) • Affiliate Nominations Strategy Helen Stevens reviewed the positions on the HL7 Board, TSC and Steering Divisions up for renewal this year. Members of the council were encouraged to identify suitable candidates from their affiliates for these positions and submit nominations. Questions may be sent to Helen Stevens (as the Council’s representative on the Nominations Committee). • HL7 Global Partnerships and Policy Discussion (Ticia Gerber) Ticia Gerber, HL7 Director of Global Partnerships and Policy gave a presentation on her role and activities, similar to that presented to the HL7 Board (see under the heading “DIRECTOR OF GLOBAL PARTNERSHIPS AND POLICY” in the report on HL7 Board activities above). • IHIC 2013 Update. Richard Dixon Hughes gave presentations on progress toward organisation of IHIC 2013 in Sydney on October 29-30. 2.17.2 ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by International Council: Issue: HL7 Australia is organising the International HL7 Interoperability Conference (IHIC 2013) in Sydney in a time slot adjacent to the ISO/TC215 meeting being planned for October 2013 and has received $US5,000 support toward this end from the HL7 International Council. There is a lot of organisation still to be completed. HL7 Australia Staging of IHIC 2013 in Sydney Action: HL7 Australia to progress the organisation of IHIC 2013 in Australia in October 2013 and get calls for papers and publicity in progress by end-June 2013. 91 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.18 JOINT INITIATIVE COUNCIL LIAISON 2.18.1 PROGRESS AT THIS MEETING As Chair of the joint initiative Council for global health Informatics standardisation (JIC), Richard Dixon Hughes was involved in a range of JIC liaison activities, principally • Representing and presenting on progress of the JIC at the “HL7 and other SDOs” session conducted by the TSC. • Informal discussions on behalf of the JIC with the CEO, CTO and members of the Board of HL7 International. Some HL7 WGMs represent an opportunity for Richard Dixon Hughes to meet face-to-face with the Lisa Spellman, head of the JIC Secretariat. This HL7 WGM followed the TC215 meeting in Mexico at which there had been a face-to-face meeting of the JIC executive, which had also been attended by HL7 representatives and the JIC Secretariat. The week after this WGM, further meetings relating to JIC activities were planned at the European eHealth week in Dublin, which was also attended by the JIC Secretariat. Under these circumstances the requirement for JIC liaison this WGM was somewhat reduced. 2.18.2 ACTIONS FOR AUSTRALIA No actions required at present. 92 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.19 MEMBERSHIP TASKFORCE PROGRESS AT THIS MEETING The former Membership Taskforce has now been replaced by a Membership Committee, which is an enduring committee of the HL7 Board. Information about the activities of the Membership Committee are reported under the heading “MEMBERSHIP COMMITTEE” in the section of this report dealing with the HL7 Board. 2.19.1 ACTIONS FOR AUSTRALIA No actions required at present. 93 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.20 MOBILE HEALTH 2.20.1 PROGRESS AT THIS MEETING This group is established to work with health care devices, EHR, Pharmacy, PHER, and Security groups to create and promote a framework and standards for mobile health. It has developed a set of scenarios that depict how mobile health can contribute to improved patient care and health outcomes: • An EHR system services (including devices and applications) that follow providers around in hospital. • Assisted independent living (of frail, elderly, those with mental difficulties) using a range of mobile devices. • Patient empowerment and support for long term condition management across a range of lifestyles. • Behavioural health support anytime, anywhere. • Providing trusted health information on child health to hard-to-reach families. It has also developed a mobile health requirements matrix for a range of health care settings to which the above scenarios can be mapped. Security of mobile health application design, implementation, testing, compliance verification and evaluation is considered increasingly important for developers and users. The group also plans to develop an FAQ wiki page to provide information on security issues relevant to mobile health applications. A project scope statement has been developed and is currently in approval phase. Target publication and annual revision dates are set to the end of each calendar year. It appears that a parallel group called Open Mobile Health (http://openmhealth.org/) has been established after 2010 to develop open standards/infrastructure to grow data standards and software to support implementation of mobile health technologies. It uses a use-case driven approach to drive the development of open standards in architecture, data and applications. It has published example polymorphic data models, schema and is building a catalogue of schema IDs, APIs and application modules to support the exchange of health data between mobile applications/devices. 2.20.2 CURRENT PROJECTS There are currently seven projects that are awaiting approval. • Project #956: Education and Communications • Project #955: Standards or Guidelines for using mobile devices in LMIC to reduce childhood mortality • Project #954: Mobile Specific Profile for PHR-S • Project #953 Conformance Spec for mobile device interface to EHR/PHR • Project #942 Medical device information guidelines • Project #941 Security Guidelines for Mobile Health Informatics Products 94 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.20.2.1 PROJECT #956 EDUCATION AND COMMUNICATIONS PROJECT OBJECTIVE Educate the industry on mHealth topic; Communicate news and updates on mHealth; Advocate on issues related to mHealth; Collaborate with mHealth groups/orgs and invite experts and leaders to share information. PROJECT ACTIVITY/ISSUES AT THIS MEETING First MH Newsletter and MH Articles were released. The need for HIT Standards in this area was noted. 2.20.2.2 PROJECT #955: STANDARDS OR GUIDELINES FOR USING MOBILE DEVICES IN LMIC TO REDUCE CHILDHOOD MORTALITY PROJECT OBJECTIVE To produce standards or guidelines for using mobile devices in LMIC to reduce childhood mortality. No further details are yet available. PROJECT ACTIVITY/ISSUES AT THIS MEETING This project being run by the MHWG LMIC sub-group is being led by John Ritter, Gora Datta and Nadine Manjaro but has get to progress significantly. Mention was made of activities related to e-Health and LMIC, notably the “Save a Million Lives” project. 2.20.2.3 PROJECT #954 MOBILE SPECIFIC PROFILE FOR PHR-S PROJECT OBJECTIVE Review the PHR-S functional model to determine how the introduction of mobile devices as actors within the model may result in changes to the model. PROJECT ACTIVITY/ISSUES AT THIS MEETING The ability of mobile health to capture environmental metrics and alerts was discussed, as was smart phones as application for improved workflow management. 2.20.2.4 PROJECT #953 CONFORMANCE SPEC FOR MOBILE DEVICE INTERFACE TO EHR/PHR PROJECT OBJECTIVE To standardize implementation guides to facilitate mobile health application development. Phase 1: Develop Conformance Specifications for Mobile Interoperability Phase 2: Develop an Implementation Guide in collaboration with the FHIR Workgroup Phase 3: Education and Industry Adoption. PROJECT ACTIVITY/ISSUES AT THIS MEETING Due to unavailability of delegates, detailed reporting was unable to be provided on this project. 95 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.20.2.5 PROJECT #942 MEDICAL DEVICE INFORMATION GUIDELINES PROJECT OBJECTIVE To publish Medical Device Information Guidelines for mobile devices – no further detail is available yet. PROJECT ACTIVITY/ISSUES AT THIS MEETING Continued work with Health Care Devices (HCD) and broad scope of Mobile Health. 2.20.2.6 PROJECT #941 SECURITY GUIDELINES FOR MOBILE HEALTH INFORMATICS PRODUCTS PROJECT OBJECTIVE To publish Security Guidelines for Mobile Health Informatics Products – no further detail is available yet. PROJECT ACTIVITY/ISSUES AT THIS MEETING Due to unavailability of delegates, detailed reporting was unable to be provided on this project. 2.20.3 2.20.3.1 OTHER NON-PROJECT WORKING GROUP DISCUSSIONS AND PRESENTATIONS EDUCATIONAL SESSION A mobile health free tutorial was attended in the context of this working group meeting. The Education working group discussed feedback amongst participants at this tutorial about the benefit of adding more technical information. Never the less this tutorial was an interesting introduction to the HL7 mobile health working group agenda. Some drivers for mobile health development were explained as including: • Correlation of mobile health with personal and electronic health records • The need to define and define the use and storing of mobile health data in the privacy, regulatory and quality domains as well as medico-legal areas • Improved patient and clinical safety • The integration with telehealth and other mobile solutions • The ever expanding focus on interoperability e.g. "smart homes" The scope and purpose of the mobile health work group does not so much seek to offer new technology but integrates work from existing HL7 areas. Examples were given to include: an EHR following patients and providers around settings, providing mobile PHR interfaces and to support ordering. Use cases for mobile health include: ward rounds, administering mediations, monitoring and recording, phlebotomy, clinical handover. Normally tied to closed networks which is an issue - open standards and open networks to get devices to all link up are needed e.g. doesn't matter where you fall or where you need the reminder - Problem : standards working in the semantic domains for interoperability - family law and divorce : both parents can log into a website to see information about a child - cross over between other sectors e.g. community services, law 96 Final Report HL7 Meeting—Atlanta, USA (May 2013) health care advice outreach for basic services - continuity of services displaced people, seasonal contract workers - Significant difference between a mobile app that only gives information to one that gives you instructions to do something. 2.20.4 ACTIONS FOR AUSTRALIA Topic Issue / Action / Recommendations for Australia Recommended for Action by Mobile Health Issue: Benefits and opportunities for mobile health include: extending provider mobility especially for community clinics, rural and remote and more mobile workforces e.g. district nurses, improving safety through identification standards, record keeping, identification of devices for supply chain management. IT-014 Standards Australia Future delegations Action: integration of mobile health within Australian context and work programs. 97 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.21 MODELLING AND METHODOLOGY 2.21.1 PROGRESS AT THIS MEETING The progress reporting is limited due to limitations of the delegation to cover this workgroup. The progress is reported under the project below. 2.21.2 CURRENT PROJECTS The following project is discussed in this section: • Project #891: FHIR Resource DSTU Ballot 2.21.2.1 PROJECT #891: FHIR RESOURCE DSTU BALLOT PROJECT OBJECTIVE This project will document the core elements of the FHIR specification, including aspects of the methodology resulting from the FHIR methodology project and combine this content with resources defined by various HL7 committee resources. This combination of materials will then be balloted as a DSTU specification. The project will also manage coordination of resolution of ballot issues amongst the disparate submitters. The project will also coordinate the collection and publication (for review) of a set of key extensions for data elements not handled directly by resources that are expected to be needed by initial implementers. Note that FHIR combines both granular and aggregated clinical concepts along with an XML-focussed, RESTful and/or SOA exchange format. The WG is considering approving FHIR as a standard for rapid adoption of RIM and CDA R3 standards. FHIR defines a set of ‘Resources’ that represent granular clinical concepts. The resources can be managed in isolation, or aggregated into complex documents. This flexibility offers coherent solutions for a range of interoperability problems. The simple direct definitions of the resources are based on thorough requirements gathering, formal analysis and extensive cross-mapping to other relevant standards. Technically, FHIR is designed for the web and the resources are based on simple XML, with an http-based RESTful protocol where each resource has predictable URL. Where possible, ensure open Internet standards are used for data representation. This project is targeted at green-field sites and offers an alternative approach to CDA as it simplifies the data into more manageable XML structures. PROJECT ACTIVITY/ISSUES AT THIS MEETING A substantiative part of the MNM FHIR sessions were related to the resolution of ballot comments submitted in the January FHIR Ballot for Comments. As has been the case at previous WGMs, FHIR continues to rapidly evolve. Notably, as knowledge and understanding of FHIR methodology has become more widespread, the nature of the discussions have become more informed and focused. The instigation of a Quality Assurance program across FHIR promises improved consistency across this evolving standard. 98 Final Report HL7 Meeting—Atlanta, USA (May 2013) FHIR does however remain a challenge for WGs who have been involved from an early stage in the development of FHIR resources, in respect that the methodology, rules of thumb and implicit best practice approaches that currently change from WGM to WGM require a review of already completed resources, and imply an element of rework. 2.21.3 ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by Modelling and Methodology & Patient Administration: Issue: FHIR Resource development requires ongoing Australian input to influence the inclusion of data elements that would be considered core for Australia. NEHTA FHIR Resources Action: Continue to support and engage Australian Subject matter experts in the development of domain specific resources including Encounter resource for community contexts. Standards Australia Future HL7 delegations 99 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.22 PATIENT ADMINISTRATION 2.22.1 PROGRESS AT THIS MEETING The Patient Administration agenda for this WGM was focused almost exclusively on FHIR resources, and resolution of FHIR Ballot comments relating to the core resources for which the PA WG is responsible. Perhaps as an indicator of the widening focus on FHIR, the WG was attended by a significantly larger contingent of vendor representatives. This included a tripling of the number of participants from EPIC, and the participation of at least one Cerner representative in every PA FHIR session. With the participation of Industry behemoths such as Cerner and EPIC, and the extensive use of at least Cerner’s software through Public Hospitals in Australian states, it is imperative that Australian requirements are tabled and argued in this forum. Attendance at this WG was limited due to the logistical shortage of attendees. Australia (via Amy Mayer) has however agreed to assist with the development of the Encounter resource for community contexts. This will be progressed in the coming weeks and months out of session and during conference calls. This is in line with the recommendation made by the delegation to the Phoenix working group meeting in January this year that FHIR Resource development needs Australian input to influence the inclusion of data elements that would be considered core for Australia. 2.22.2 CURRENT PROJECTS The following project is reported on in this section: • Project #925: Development of FHIR Resources 2.22.2.1 PROJECT #925 DEVELOPMENT OF FHIR RESOURCES PROJECT OBJECTIVE This goal of this project is to identify and define an initial set of foundation FHIR resources within the Patient Administration Domain. PROJECT ACTIVITY In addition to FHIR Ballot reconciliation, significant progress was made in the discussion and evolution of the Visit/Encounter resource, and the separation of the concepts of a planned attendance (i.e. an Appointment) and the actualisation of an attendance (i.e. an Encounter). The Encounter resource is designed to accommodate multiple domains including InPatient admissions, Outpatient and Community visits, so its scope is significant. An extensive debate was had amongst the EPIC, Cerner and Australian representatives on the granularity of the Encounter resource elements. There was considerable push from the US multinationals for preserving existing HL7 v2 concepts and workflows, while the Australian representative argued for structures that better supported Australian federal and state based reporting requirements. This remains a difficult argument for Australia, as from a FHIR development perspective EPIC and Cerner represent a sizeable portion of the worldwide installed base of Hospital Information Systems, and on the FHIR 80/20 rule this implies a degree of influence on outcomes that is not necessarily in Australia’s long term interests. 100 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.22.3 ACTIONS FOR AUSTRLIA Topic Issue/Action/Recommendations for Australia Recommended for Action by Patient Administration: Issue: FHIR Resource development needs Australian input to influence the inclusion of data elements that would be considered core for Australia (as noted previously in Phoenix 2013 Meeting report). NEHTA FHIR Resources Action: Engage Australian Subject matter experts in the development of domain specific resources. Standards Australia Future HL7 delegations 101 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.23 PATIENT CARE 2.23.1 PROGRESS AT THIS MEETING There were a number of important Patient Care Workgroup Activities during the Atlanta meeting. These activities can be broadly divided into two categories: Patient Care projects and FHIR resource development. 2.23.2 CURRENT PROJECTS The following projects are reported on in this section: • Project #675: Allergies and Adverse Reactions • Project #932: Care Plan Initiative • Project #924: Care Coordination Services Functional Model 2.23.2.1 PROJECT #675: ALLERGIES AND ADVERSE REACTIONS PROJECT OBJECTIVE This project is about harmonising a number of different models that are in use, or are being considered in various parts of the world, and to improve on the existing DSTU artefacts previously produced by the Patient Care (PC) WG. The project team conducted detailed comparative analysis of care plan models submitted from around the world including the US, Canada, Sweden and Australia (NEHTA). This ongoing work aims at developing a DAM to ensure that all requirements are met in the redevelopment of the existing DSTU package content that included the allergy/intolerance model. The current Allergy/Intolerance topic DSTU expired in June 2012. A request had been made and approved to extend the current DSTU for another 2 years. The project team co-led by Stephen Chu of Australia and Elaine Ayres of the US National Institute of Health, and supported by a number of contributors, including a US-based allergist, has been developing a DAM to define and model the requirements thoroughly, assess the adequacy and currency of the current DSTU, and the extent of work required to be done on the DSTU. PROJECT ACTIVITY/ISSUES AT THIS MEETING The DAM package was revised in accordance with the ballot disposition decisions arising from the reconciliation process after the first informative ballot in January 2013. It has undergone a second informative ballot in May 2013. A much smaller number of Ballot comments were received, which were addressed during reconciliation discussions at the WGM. A new project is in the process of being established to develop a logical model and full HL7 v3 artefacts to support documentation and exchange of allergy/intolerance lists and adverse reaction information and reporting. A new project scope statement (PSS) has been development that was endorsed by Patient Care, Orders and Observations (OO) and the Clinical Decision Support WG joint meeting. The Pharmacy WG is also a co-sponsor of this project. The group is currently reviewing the PSS and conduct an e-voting process to formalise the endorsement of this project. The pharmacy WG is in principle supportive of this project but has requested clarification of the boundary and scope of a couple of proposed artefacts. This project has attracted attention and interest from the US Office of National Coordination of IT (ONC), which has engaged a consultant from Accenture to work on this topic. More details of this project are available on the Patient Care wiki: http://wiki.hl7.org/index.php?title=Allergy_%26_Intolerance [accessed: 16 May 2013] 102 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.23.2.2 PROJECT #932: CARE PLAN PROJECT OBJECTIVE The Care Plan Topic is one of the rollouts of the 2007 version of the Care Provision Domain Message Information Model (D-MIM). The Care Plan is a specification of the Care Statement (now replaced with Clinical Statement) with a focus on defined Acts in a guideline and their transformation towards an individualized plan of care in which the selected Acts are added. Generally a care plan greatly aids the team (responsible parties) in understanding and coordinating the actions that need to be performed for the person. The objective of this project is to develop a domain analysis model (DAM) to support the definition of: The care plan structure covering key components such as health concern, risks, goals, intervention, outcome reviews, etc., to support effective management action plans of various conditions identified for the target of care. It is the structure in which the care planning for all individual professions or for groups of professionals can be organized, planned, communicated, implemented and checked for completion against predetermined goals and planned actions. Care plans also permit the monitoring and flagging of unperformed/overdue activities and unmet goals for later follow up. The behavioural requirements to support effective implementation of the care plan and the care coordination required. The Care Plan project was established in 2011 with Stephen Chu (Australia) and Laura Heermann Langford (Intermountain Medical Centre, USA) as co-leads. The goal is to complete a DAM to describe the context of the act of care planning and artefacts created during the process. PROJECT ACTIVITY/ISSUES AT THIS MEETING The project plan of submitting the Care Plan DAM package for first informative ballot in May 2013 did not eventuate due to the complexity of use cases/storyboards and the higher than expected number of comments from various interested parties, in particularly the care plan structure model. A decision was made to defer the first informative ballot to September 2013. The Care Plan Project continues to receive a significant amount of engagement from the ONC S&I framework Longitudinal Care Coordination (LCC) workgroup. The ONC LCC group has established a tiger team to work closely with the care plan project and care coordination functional model teams. A number of intensive email changes and conference calls were initiated between the LCC group/tiger team and the Care Plan project team to harmonize business/clinical terminology and care plan structure requirements. More information about the ONC initiative on care plan is available on the LCC website: http://wiki.siframework.org/Longitudinal+Coordination+of+Care+%28LCC%29 [accessed on 16 May 2013] Further harmonization conference calls between the LCC tiger team and the care plan project team have been planned for Wednesdays at 5:00pm US Eastern to accommodate Australian (Stephen Chu) time constraints. The first call is scheduled for Wednesday 15 May. The care plan project team members (e.g. Laura Heermann and Susan Campbell) are also working closely with IHE Patient Care Coordination (PCC) on its technical profile for care plan. The objective is to ensure harmonization of the structural and behavioural models produced by the two groups. More information on the care plan project is available on the Care Plan wiki: http://wiki.hl7.org/index.php?title=Care_Plan_Project_2012 [accessed: 18 May 2013] 103 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.23.2.3 PROJECT #924: CARE COORDINATION SERVICES FUNCTION MODEL PROJECT OBJECTIVE The Care coordination Services (CCS) Functional Model (FM) project is a Patient Care project with Service Oriented Architecture (SOA) and Clinical Decision Support workgroups as co-sponsors. Its objective is to provide a platform independent model of capabilities to support care coordination and collaboration among a multi-disciplinary care team consisting of members from either the same or different organizations (e.g. primary care clinic, home care, allied health professionals, hospital, skilled nursing facility, etc.) The service specification will support real time conversation between participants based on a shared coordinating care plan. All authorised stake holders and collaborators in a patient’s care can participate in planning, initiation, updating the care plan contents including health concerns, health risks, care barriers, and perform reviews (goals, plan effectiveness, outcomes) where appropriate/required. The CCS interface will expose a consolidated view to all participants and maintain a shared context. The CCS FM builds on the business/clinical requirements and the behavioural requirements defined in the care plan DAM project. The ultimate object of the CCS FM is to support the design and development of a set of CCS technical specification to support care services coordination. The technical specification will be developed by the Object Management Group (OMG) in collaboration with HL7 SOA and Patient Care WG. PROJECT ACTIVITY/ISSUES AT THIS MEETING The CCS FM project team consists of modelling leads from the SOA workgroup, the co-leads from the care plan project and a number of active Patient Care members. The development process of the CCS FM continued to provide very useful feedback that resulted in refinements of the Care Plan structural model design. Acknowledging the importance and significant interest in the care plan and its related project, the ONC LCC tiger team met almost weekly leading up to the closure of the CCS FM ballot to review the ballot contents and provide feedback/comments to both the care plan and CCS project teams. The CCS FM has gone through an informative ballot in the May 2013 ballot cycle. It attracted approximately 80 ballot comments, majority of which were from the ONC LCC tiger team. Ballot reconciliation was initiated in the Atlanta meeting and will continue via post Atlanta meeting conference calls. The plan is to fully address the ballot comments and to move onto DSTU ballot in September 2013. More details of this project are available at this wiki page: http://wiki.hl7.org/index.php?title=Care_Coordination_Capabilities; and http://hssp-carecoordination.wikispaces.com/home [both accessed: 20 May 2013] 2.23.2.4 PATIENT CARE FHIR RESOURCES The FHIR modelling team developed a number of Patient Care WG related FHIR resource models: • Allergy/intolerance • Adverse reaction • Care plan 104 Final Report HL7 Meeting—Atlanta, USA (May 2013) • Family history • Observation • Problem • Procedure While they are very useful resources, with the exception of a couple, they are essentially too simplistic. Members from Patient Care and other groups such as clinical genomics criticised some of the FHIR resources as “disconnected from clinical reality”. The Patient Care WG had a joint meeting with FHIR modelling team in Atlanta to review and improve some of the models. Engagement via email will continue leading up to the first ballot of these FHIR resources in September. Details on Patient Care FHIR resources are available at the FHIR wiki: http://hl7.org/implement/standards/fhir/index.htm [accessed: 20 May 2013] 2.23.3 ACTIONS FOR AUSTRALIA Topic Issue / Action / Recommendations for Australia Recommended for Action by Patient Care: Issue: Ongoing work on allergy/intolerance and adverse reaction in Care Plan DAMs needs to be noted by relevant IT-014 groups and considered for incorporation into relevant Australian activities. IT-014-06-04 Allergy/Intolerance and Adverse Reaction, Care Plan DAMs; CCS FM; and FHIR resources models IT-014-06-05 Action: Allergy/Intolerance, Adverse Reaction, and FHIR resource Models are of interest to and should be monitored by IT-014-06-04, IT-014-06-05, IT-014-06-06 and IT-014-13. IT-014-13 Care Plan and CCS are of interest to IT-014-06-06, IT-014-09 and IT-014-13. Australian eHealth All these projects are of interest to NEHTA and PCEHR projects. IT-014-06-06 Communities 105 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.24 PHARMACY 2.24.1 PROGRESS AT THIS MEETING There was no ballot submission or new project for the Pharmacy workgroup at the Atlanta meeting. For this meeting, the discussions focused on a number of topics: The modelling of access in the [medication administration] Device model, Medication profile, FHIR resources for pharmacy, and ISO WG6 Updates. 2.24.1.1 ACCESS IN DEVICE MODEL There is a requirement to indicate that a medication is administered through an access port (e.g. an intravascular catheter such as a subclavian line) connected to an IV infusion pump. The intravascular catheter is a multi-port access device where each port is designated by a Port ID. Currently there is some issue/confusion in the pharmacy DMIM (domain messaging information model) in how the Device Entity and Access is modelled. It was agreed that the relevant DMIM and other appropriate RMIM (refined messaging information model) would be updated as part of the DMIM/RMIM synchronisation activities. 2.24.1.2 MEDICATION PROFILE There were extensive debates on what actually constituted a medication profile. An extensive literature search by a number of active members of the Pharmacy WG members (including Stephen Chu and Julie James) could not identify any known definition of medication profile. The group agreed that it would be difficult to clearly identify what constituted medication profile in the absence of any clear definition. Discussions shifted to identifying what would the business/clinical process be and what useful building blocks would be. The group also looked at an example of “medication profile” from the Saskatchewan province of Canada and concluded that it was more likely to be a medication list. Based on the discussions at the Baltimore meeting, the group agreed that if a set of existing queries previously defined by the group were to be used to retrieve medication data: • Medication order query • Medication dispense/supply query • Medication statement query The queries would return one or more medication lists and not necessarily a ‘medication profile’. The group agreed that this was a good starting point. It was suggested that all possible query parameters should be identified to allow implementers to define different combinations of required queries. The result of the queries would bring back the building block information required for the medication list or profile to be constructed. It was also agreed that the resulting information constructed from the query results should be extended to something that could be used in C-CDA. The next step is to build the storyboard narrative to reflect the types of query required. 106 Final Report HL7 Meeting—Atlanta, USA (May 2013) The plan is to prepare the content for September 2013. 2.24.1.3 FHIR PHARMACY RESOURCES Five pharmacy FHIR resources have been developed: • Medication • Medication prescription • Medication dispense • Medication administration • Medication statement These resources were modelled by one of the Pharmacy Co-Chairs who are also a member of the FHIR team. Nevertheless, a number of issues were identified and discussed/debated. For example: • Medication resource needs to be added to medication dispense. • Medication administration requires inclusion of person (e.g. patient, relative, and carer) who administer the drug. • Decide whether dose titration and “rescue agent” in chemotherapy prescribing and administration are in scope for FHIR (decision: probably out of scope). • Decide whether device and medication should be separate resources. In the UK, a medication is defined as a substance that has a pharmacological effect on a person. Substances like artificial teardrops that only have a physiological effect cannot be classified as a medication. This regulatory constraint causes a problem in recording administration of such an agent. There was a strong argument to use “device” as a generic concept covering both pharmacological and physiological agents. This approach is likely to cause confusion in other realms where device is taken to mean infusion pump, monitors, etc. There was no conclusion and discussion is likely to continue. More information about pharmacy FHIR resources can be located on the FHIR resources wiki: http://hl7.org/implement/standards/fhir/ [assessed: 20 May 2012] 2.24.1.4 ISO WG6 UPDATES ISO Workgroup 6 is working on a number of specifications and new work item proposals: Dose Syntax: This is a business requirement document on dose syntax and not a technical specification on how dose syntax should be structured to support complex prescribing. There appeared to be little progress on this work item since the last meeting. It was mentioned that the requirements were leaning towards the NCPDP (the US National Council for Prescription Program). Members expressed concern in the change in direction. Confirmation would be required. Drug Dictionary: This work item is based on drug dictionary work done in Singapore. It appeared that this item was still in a brainstorming mode. The project goal is to establish a comprehensive list of requirements for drug dictionary. A new work item proposal is expected to be published in June 2013. Prescription Standard: This specification is based on previous work done by the Netherlands. Members at the HL7 meeting expressed significant concern with this project as prescription practices are heavily regulated by legislation from different realms/jurisdictions. A specification based on Dutch experiences 107 Final Report HL7 Meeting—Atlanta, USA (May 2013) would be unlikely to have international applicability and would potentially be in conflict with existing international standards. It is expected that a draft specification will be available for comment in June. Dispense Record: This is also based on work from the Netherlands. The focus of this work item is likely to be on functional requirements and not on content or technical specification. The proposal is also expected to be available for comment in June 2013. Administration Record: This will be another new work item proposal. Information is scarce on this work item. HL7 Pharmacy is waiting for more information from WG6. Compound Medicine: This is intended to cover magisterial/extemporaneous (off-label) preparations. Again the scope of this proposal/project is unclear at this point in time. Electronic Patient/Consumer Drug Information: This new proposal/project aims to replace the current paper version of consumer drug information by an electronic publication. HL7 Pharmacy members questioned the viability of such a project as many international realm’s/jurisdiction’s legislation require such materials to be provided as hard copy. It appears that ISO is working with European legislators to overcome this issue. It appears that WG6 is establishing a very large number of work items without much consultation with other international standards bodies. While many of the items listed are still in a “brain-storming” stage, they triggered serious concerns and questions on the utility of such projects and the resources available to complete these projects. HL7 Pharmacy, IHE Pharmacy and ISO WG6 will meet in an out-of-cycle pharmacy meeting in Europe in June. These projects will be discussed further at this meeting. 2.24.2 ACTIONS FOR AUSTRALIA Topic Issue / Action / Recommendations for Australia Recommended for Action by Pharmacy: Issue: The medication profile work is of high importance to Australia. IT-014-06-04; IT-014-06-06 and IT-014-13, NEHTA and PCEHR projects in particular should monitor and provide relevant input to this activity. IT-014-06-04 HL7/IHE Medication Profile ISO/TC 215 WG6 projects Action: The ISO WG6 activities remain a concern to other international groups. There needs to be a closer link between the Australian delegate to ISO WG6 and IT14-x groups to ensure visibility of the ISO projects. IT-014-06-06 IT-014-13 Australian eHealth communities 108 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.25 POLICY ADVISORY COMMITTEE 2.25.1 PROGRESS AT THIS MEETING Richard Dixon Hughes attended a face-to-face meeting of the board-appointed Policy Advisory Committee (PAC). Matters addressed included: • Follow-up on recent HL7 responses to US government agencies in respect of proposed regulation. • A meeting with Ticia Gerber concerning her role and functions and her plans to leverage and complement the skills and capabilities of the PAC. More information on Ticia Gerber and her role is reported under the heading “DIRECTOR OF GLOBAL PARTNERSHIPS AND POLICY” in the section of this report on the HL7 Board activities. • The potential to expand the PAC to include more non-US representatives and to prepare materials relevant to policy in other realms (particularly Latin America) was discussed. This potential will be explored further with the International Council, noting that the PAC is a board-appointed committee. It was noted that the CQI committee will be considering whether it wishes to lead the drafting of a response to the US-CMS Notice for Proposed Rule Making (NPRM) on US Hospital Inpatient Prospective Payment System for Acute Care Hospitals (IPPS) which needs to be submitted by 25 June. The work of the PAC will continue by the normal regular monthly teleconferences. More information on the PAC and its activities may be found in the report of the meeting between the PAC Chair and the International Council in section 2.17.1. 2.25.2 ACTIONS FOR AUSTRALIA No actions required at present. 109 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.26 SECURITY 2.26.1 PROGRESS AT THIS MEETING The majority of the security sectors were dealing with ballot reconciliation and new project development. 2.26.2 CURRENT PROJECTS The following projects are reported on in this section: • Project #646: Security and Privacy Ontology • Project #823: Fast Health Interoperability Resource (FHIR) • Project: # 529 Composite Security and Privacy Domain Analysis Model (DAM) • Project #1006: HL7 Data Segmentation for Privacy (DS4P) Implementation Guide • Project #914: HL7 Security Service Oriented Architecture Domain Analysis Model (SSOA DAM). 2.26.2.1 PROJECT #646: SECURITY AND PRIVACY ONTOLOGY PROJECT OBJECTIVE This project will develop a domain ontology encompassing the healthcare IT security and privacy domains providing a single, formal vocabulary embodying the concepts in each domain as well as concepts shared between the two. The concepts identified and defined in this ontology will be primarily drawn from those concepts contained in the Security and Composite Privacy DAMs. The concepts in this ontology will be extended in order to bridge to standard ontologies in associated domains such as enterprise architecture, clinical care and biomedicine. This project will support modelling and analysis of the unified Security (privacy DAM), and identify areas where reference value sets are needed. Existing terminologies, where applicable, will be utilised and/or extended. Where appropriate terminologies are not available, they will be developed to meet the needs of the models. Concepts covered by security and privacy domains that are relevant to this ontology include (but are not restricted to) security and privacy policies, consent directives, and access control. PROJECT ACTIVITY/ISSUES AT THIS MEETING The ballot resulted in an achieved quorum 75% approval 51 to 17. Several comments are to be applied at reconciliation. The majority of the meeting time was used to reconcile and resolve the ballot comments. 2.26.2.2 PROJECT #823: FAST HEALTH INTEROPERABILITY RESOURCE (FHIR) PROJECT OBJECTIVE FHIR combines both granular and aggregated clinical concepts along with an XML-focussed, RESTful and/or SOA exchange format. The WG is considering approving FHIR as a standard for rapid adoption of RIM and CDA R3 standards. FHIR defines a set of ‘Resources’ that represent granular clinical concepts. The resources can be managed in isolation, or aggregated into complex documents. This flexibility offers coherent solutions for a range of interoperability problems. 110 Final Report HL7 Meeting—Atlanta, USA (May 2013) The simple direct definitions of the resources are based on thorough requirements gathering, formal analysis and extensive cross-mapping to other relevant standards. A workflow management layer provides support for designing, procuring and integrating solutions. Technically, FHIR is designed for the web and the resources are based on simple XML, with an http-based RESTful protocol where each resource has predictable URL. Where possible, ensure open Internet standards are used for data representation. This project is targeted at Green field sites and offers an alternative approach to CDA as it simplifies the data into more manageable XML structures. PROJECT ACTIVITY/ISSUES AT THIS MEETING One of the issues raised was the attributes associated with FHIR. There is discussion on the attributes location in a general resource. There is concern regarding the clinical attributes and how these are dealt with in the general FHIR and security and privacy are treated in the same way. In FHIR’s purposeful light weight design, items that are part of the resource (like attributes) are not part of the resource but extensions. John Moehrke, on behalf of the Security WG has been involved. There are issues in relation to how FHIR models the security, privacy and provenance that are integral to the work occurring in CBCC and SEC projects. Further information can be found on this discussion in the CBCC minutes from the 2013 Atlanta Working Group Meeting. 2.26.2.3 PROJECT: # 529 COMPOSITE SECURITY AND PRIVACY DOMAIN ANALYSIS MODEL (DAM) PROJECT OBJECTIVE This project is intended to create and ballot a single HL7 DAM integrating both security access control and privacy information models. PROJECT ACTIVITY/ISSUES AT THIS MEETING Composite security and privacy DAM/Information: It was discussed that there is a need to review and apply changes and it is related to several items. The work may need to re ballot and enumerate the changes. The aim will be to engage the HL7 community in reviewing this as a new artefact, although being cognizant of the issues this may raise. The changes may include adding the Healthcare Classification Scheme labelling and elaborating on the contextual operations. This will require amendments to the scope statement and will include the harmonization of the DAM and ontology as the DAM is the basis of all the projects. The work needs to more closely adapt to the DAM, and because conceptually the DAM leads to the ontology. There is also a possible review of the DAM required in light of the other projects currently underway, with a possible re-ballot in September 2013. Whilst the DAM is a static picture currently and provides the classes, but the attributes have not yet been added. Whilst the baseline classes will not change, we also need to see how the run time version will look when labels are integrated into the whole picture. It is also important to qualify the connections between the attributes and classes as the problem is the people who use the dam are not necessarily the people who are sitting at this table; they are using this as an IG. They do not take the management approach and it becomes 'when talking implementation, it doesn’t match the model that you need. The forthcoming changes are a refinement and the implementer needs more detail. Bernd Blobel will provide details of who is using the DAM in the EU and possible use cases. In 111 Final Report HL7 Meeting—Atlanta, USA (May 2013) the original scope statement it was proposed that the DAM would be used by SDOs as well, as a representation of particular requirements, based on PMAC. The potential issue is that if the DAM is highly defined for HL7, then it cannot be used for other SDOs. We have several DAMs that are highly specialized that are not associated with a domain and this is problem for the standards community in cross-use of standards. It was proposed that the group also look at the mandatory NIST security and privacy goals, aligned with the dam and where the security is aligned with privacy (controls). NIST Standards link: http://wiki.hl7.org/index.php?title=HL7_Security_Document_Library 2.26.2.4 PROJECT: #1006: HL7 DATA SEGMENTATION FOR PRIVACY (DS4P) IMPLEMENTATION GUIDE PROJECT OBJECTIVE This project is intended to create and ballot a single HL7 DAM integrating both security access control and privacy information models. The Office of the National Coordinator DS4P Implementation Guide (IG) will provide the core input into the HL7 DS4P IG project. The project scope is the publication of a U.S. realm DS4P DSTU specification as an exemplar for an IG that could be used by other realms. This will entail: Developing: • A US profile of the DS4P transport for email/XDM, SOAP/XDR and CDA content. • Conformance statements and constraint mechanisms required for publication are included in the HL7 DS4P IG. Ensuring that: • The IG identifies the normative standards that it constrains, and a description of how the IG is compliant with its base normative standards. • The US realm constrained IG is clearly derived from the current or updated ONC DS4P IG and IG derivatives per exchange architecture. • The IG includes adequate implementation guidance to developers. • All IG non-HL7 standards (including vocabulary) are identified and any gaps addressed, e.g., as future harmonization proposals or as requests to appropriate SDOs. Alignment with: • Other HL7 Security, CBCC, Structured Documents, and SOA Work Group standards is specified as appropriate, e.g., relationship to the: • Security Work Group HL7 Security and Privacy DAM and Healthcare Privacy and Security Classification System. • CBCC Work Group Privacy Consent Directive and CDA R2 Implementation Guide DSTU/Normative. • Structured Document Work Group CDA, CCDA, and where appropriate to CDA R3 as an emerging standard. • SOA Work Group PASS artefacts. • Applicable IHE standards in addition to the base XD* metadata and exchange protocols, such as ATNA and XUA. 112 Final Report HL7 Meeting—Atlanta, USA (May 2013) • Applicable ONC Standards and Interoperability standards, such as Direct exchange protocol. PROJECT ACTIVITY/ISSUES AT THIS MEETING Following the work that has been undertaken in piloting the DS4P project by the US Department of Veterans Affairs (VA), the Substance Abuse and Mental Health Administration (SAMHSA), and Jericho Systems, this new project was developed at this working group meeting. The need for the project is that some health data requires special handling according to law, organizational policies, or patient preferences. For appropriate sharing of health information to occur, a patient must trust that a provider organization will properly handle their health data, and disclosing organizations must have confidence that recipients will follow privacy protections according to any special handling instructions. In order to facilitate this secure and trusted exchange, data needs to be segmented and assigned specific privacy controls. Data segmentation is “the process of sequestering from capture, access, or view certain data elements that are perceived by a legal entity ac, institution, organization or individual as being undesirable to share,” (Melissa Goldstein and Alison Rein, make reference).See Coleman, Johnathan. "Segmenting Data Privacy: Cross-industry Initiative Aims to Piece Out Privacy Within the Health Record." Journal of AHIMA 84, no.2 (February 2013): 34-38. The proposal is for the Implementation Guide to be put up for a normative ballot in January 2014. The project includes collaboration with IHE, ONC - Standards and Interoperability Framework (S&I) artefact, and OASIS - XACML / SAML artefacts. Also, the project will leverage the ONC DS4P IG, but will incorporate HL7 IG required information. Although this project is initially for the US Realm, a global version will be developed in tandem to minimise the effort required to extend this to other realms in the shortest possible timeframe, as and when it is required more globally. Australia is currently not in a position to implement DS4P and Japan indicated that it would want to create its own framework. If Japan were to adopt a DS4P approach, it would not work on a profile of the DS4P because core parts of it would be replaced with Japan's workflows and Japanese clinical document standards. Data Segmentation is: Process of isolating from capture, access or view certain data elements that are perceived by a legal entity and institution. The discussion is about whether or not the detailed clinical models should have specific tags i.e. security labels bind clinical metadata to patient consent. These are implemented dynamically when the information is exchanged. E.g. the synergy is layered between the system functions. The premise is to keep the clinical and security models separate. Data modelling needs contribution because adding security and privacy to the metadata is how would changes in security and privacy metadata be handled? E.g. a sensitive condition is no longer considered sensitive, a privacy policy is pre-empted, or a privacy consent directive is revoked or expires? Provenance of the data is separately maintained (some standards W3C are coming up in relation to provenance). Health Care Security Labels (HL7 Healthcare Classification Systems) derived from NIST FIPS PUB 188 Security Labels which states how security labels should be defined and used. Integrity is different from provenance – integrity is a quality of the information, provenance is history and ownership – attribute of the data - similarity groups together because the integrity is linked to the attestation of the document. 113 Final Report HL7 Meeting—Atlanta, USA (May 2013) ACTIONS FOR AUSTRALIA Topic Issue / Action / Recommendations for Australia Recommended for Action by Security: Issue: The development of the Security implementation guides needs wider than US input. HL7 Australia HL7 DS4P IG 2.26.2.5 Action: It is important that Australia contribute to the development of this project to ensure its companion work is suitable for Australia in the future. IT-014-04 PROJECT: # 914 HL7 SECURITY SERVICE ORIENTED ARCHITECTURE DOMAIN ANALYSIS MODEL (SSOA DAM) PROJECT OBJECTIVE The goal of the HL7 Security SOA Architecture project is to develop a SAIF compliant Domain Analysis Model for a security service oriented architecture, which will be the conceptual information and behavioural foundation for past and future HL7 Security and Privacy Services artefacts. The input for the SSOA DAM: HL7 Composite Security and Privacy Domain Analysis Model R1 DSTU - HL7 RBAC Catalogue - HL7 Consent Directive CDA Implementation Guide DSTU - HL7 Security and Privacy Ontology - HL7 Security and Privacy related vocabularies - HL7 v2 Confidentiality Codes and v3 Harmonization - HL7 Healthcare Security and Privacy Classification System - V3_PASS_AUDITSERV_R1_D1_2010SEP.pdf - PASS Alpha - Access Control Conceptual Model Release 1.0 - Post-Ballot Reconciliation.pdf - PASS Alpha - Access Control - Domain Analysis Model - Draft 0.1.doc - PASS Alpha - Access Control - Functional Model - Draft 0.1.doc - Data Segmentation for Privacy Implementation Guide - Query Health Envelope - VA Security Service Logical Model support for Data Segmentation and Health Care Security and Privacy Classification System - VA VAPii Reference Model - FHIMS - OASIS XSPA - WS* - OAuth 2.0 PROJECT ACTIVITY/ISSUES AT THIS MEETING This project has been deferred. The next milestone is now 2016 Jan WGM, and Project End Date 2018 Sept WGM. This is because there are no resources currently to undertake the project. 2.26.3 2.26.3.1 OTHER NON-PROJECT WORKING GROUP DISCUSSIONS AND PRESENTATIONS EDUCATION The free education session that was run for was well attended with 17 people attending the session. There were presentations on consent directives for CDA, HIMSS DS4P pilot and video, FHIR Security and audit logging to support security surveillance. 2.26.3.2 REALM REPORTS AND PRESENTATIONS ISO A full report on the ISO WG4 Security meeting was presented by Lori Forquet. The slides are available from the ISO website. 114 Final Report HL7 Meeting—Atlanta, USA (May 2013) JAPAN Japanese Association of Health Care Information Systems (JAHIS) industry has 357 companies (90% of all Japanese companies). One business section is to develop standards: • Data exchange protocols (HL7) • Technical reports • Security standards • Patient safety In 2010 a new IT strategy was launched. JAHIS is part of the committee that is implementing this strategy. Aim to bring ‘my hospital everywhere’. Japanese PHR system is dependent on the patient. The patient entrusts the clinical information bank with his/her information. The patient will allow the access to a specific service provider, e.g. hospital, fitness club. JAHIS is HL7 Japanese Realm, and has published many profiles using HL7 v2.5. There is one Japanese certificate authority for health professionals. At the moment a list of all healthcare service providers so the patient can tick which ones will have access (permission table). An in-depth discussion regarding digital signatures and partial signatures was undertaken. Every provider has a government issued JPKI from Japanese national Certificate Authority. Patients use at 3rd party PKI. Patient can choose the permission table in the PHR to allow a service provider to access the patient's PHR. Transport is a web service. Currently there are detached (file CDA and a separate signature file), enveloped (signature is within the CDA file) and enveloping (CDA and signature part in one file) signatures. IHE use cases are the way that detached signature is good enough. Japan uses the permission table in the PHR. In the future, that the permission tables would be managed centrally so that any organization meeting the clearance would have access to the patient's PHR rather than having the patient directly involved in authorizing each service provider. Japan uses all three types of digital signature. HL7 Japan has developed a CDA for prescriptions rather than phone/fax. Japan requires prescriptions in a document form. Use has Patients prefer paper prescriptions. SAUDI ARABIA (LORI FOUQUET) Saudi Arabia will use a national PKI, which is encouraged, but not mandated. It is not specific to healthcare. Providers and organizations will be issued PKI, but no plans for provisioning patients at this time. The 115 Final Report HL7 Meeting—Atlanta, USA (May 2013) Saudis are developing a provider registry and establishing professional roles. GE Healthcare has the project management contract for the analytics and specification development Most of the information is highly qualified. The focus is on health information exchange, the level of information is primarily investigating what will go into policy. The policies have not yet been approved. They are looking at an IHE federation and it will likely be centralized, but if a hospital system is purchased, with local repository options, it will not be precluded. Approximately 60% of healthcare is administered publicly, 20% through national guard/government base and another 20% in the private sector. Primary care is only dealing with initial situations, anything more than that goes to hospital (specialist). The delivery system is unique. It is very green field, not HIPAA, there is no privacy legislation but the system is using this exercise whether they should instantiate or keep in their very policy. Saudi Arabia has identified information that is sensitive mental health, substance abuse and HIV. Even though a person can opt in or out, the HIE is an opt-out, so all information goes in, but patient can opt out, but will not hold ability to break glass. The healthcare providers feel very strongly on being able to treat the patient. Opt out is not total out because a clinical override also applies. There is a disclosure in education to patients, they have not had define a policy on where and not to disclose things, but they will mark HIE as part of the exchange. They have put into effect a solution to the patient identity problem as everyone has a unique ID. The person receives no healthcare until you have a national healthcare ID. At birth a newborn gets an id. There are not a lot of legacy systems to worry about backwards compatibility as there are for other countries worldwide. 116 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.27 SERVICES ORIENTATED ARCHITECTURE 2.27.1 PROGRESS AT THIS MEETING Progress at this meeting is detailed through the current working group projects that are summarised below. Note that two projects were submitted for ballot comment in this cycle, namely Healthcare SOA Service Ontology and Patient Care Co-ordination Service (CCS) Project. Both of these projects were successfully balloted. In addition, three new projects within the SOA WG were proposed, namely Unified Communication Service, Pub/Sub Service and Ordering Service. Note that the action items were slightly updated from the previous WG meeting to reflect progress since that meeting. 2.27.2 CURRENT PROJECTS The following projects are discussed in this section: • Project #863: Cross- Paradigm Interoperability Implementation Guide for Immunization. • Project #628: Healthcare SOA Service Ontology. • Project (NEW): ORDERING SERVICES INTERFACE SPECIFICATION PROJECT. 2.27.2.1 PROJECT #863: CROSS- PARADIGM INTEROPERABILITY IMPLEMENTATION GUIDE FOR IMMUNIZATION PROJECT OBJECTIVE This is a new joint project with the SOA and ArB WGs and is co-sponsored by the CDS and PHER WGs. This IG will explore the SAIF methodology to show how various HL7, IHE and OMG immunisation-related artefacts can be deployed to satisfy immunisation interoperability use cases. Additionally, it will provide feedback to the MnM SAIF Implementation Guide project regarding artefacts and governance necessary to develop this project’s deliverables. It will build upon existing artefacts rather than create anything new. Previous work includes: • The Practical Guide to SOA in Healthcare Part II: the Immunization Case Study; • The Immunization DAM; • v2 immunization messages; • v3 POIZ messages; • v3 Care Record document; • v3 Care Record message; • vMR; IXS (Service Functional Model and Technical Specification); • RLUS; • DSS; • hData; • Arden Syntax; • GELLO; • IHE profiles including PIX, PDQ, and Immunisation Content; and 117 Final Report HL7 Meeting—Atlanta, USA (May 2013) • The IHE SOA White Paper. The scope of the immunisation use case will be limited to use cases specifically related to interoperability, primarily patient identification, immunisation data exchange and decision support (recommendations, adverse reactions, contraindications). Additional information can be located at: http://hssp.wikispaces.com/Cross+Paradigm+Interoperability+Implementation+Guide+for+Immunization [Accessed 20, January 2013]. PROJECT ACTIVITY/ISSUES AT THIS MEETING The focus of the project at this meeting was in demonstrating how the OMG Model-Driven Message Interoperability (MDMI) specification can be used to support automated mapping between different information models, e.g. between V2 and CDA artefacts, or between FHIR and C-CDA. MDMI is a generic mapping approach and the tooling was developed as part of the Model Driven Health Tools (MDHT) project. Although this specific X-paradigm project is scoped by the immunisation use case context, it can be used to support mapping between any two messaging payload types. For example, as discovered in the joint SOA/PHER (Public Health and Emergency Response) meeting, an interesting application could be mapping between OASIS Common Alerting Protocol (CAP) used for different types of alerts for emergency management, and HL7 V2 messages of relevance for PHER. The X-paradigm project uses the SAIF matrix to highlight different concerns for the mappings being developed. The SAIF matrix includes both the MDMI components such as Clinical Referent Index (which is a clinical refinement of the MDMI referent index used to support any type of message mapping), and the HL7 information models such as C-CDA models, V2 models, FHIR models etc. The immunisation DAMs are positioned in the conceptual perspective. It was recognised that this project provides significant value to HL7 and the project was encouraged to continue on its path in line with the Project Scope Statement (PSS) direction. In case of possible extensions, the project would need to advise the TSC or issue another PSS with a new scope. 2.27.2.2 PROJECT #628: HEALTHCARE SOA SERVICE ONTOLOGY PROJECT OBJECTIVE This project aims to develop a ‘Health Interoperability Service Ontology’ encompassing the description and classification of healthcare-oriented SOA services into a single, formal vocabulary. The concepts identified in this ontology will be derived from several sources including, but not limited to, the SAIF, the SOA WG Roadmap, and service capabilities identified in the HL7 EHR Functional Model. The concepts in this ontology will be extended to bridge standard ontologies in associated domains such as enterprise architecture, clinical care, and biomedicine. The project will: • Identify where reference value sets are needed; • Collect and/or develop use cases to define the objectives of the Healthcare SOA Terminology work; • Assess existing SOA ontologies for applicability to meet the needs of the healthcare domain, and recommend the best-fit for HL7; and • Enhance/extend, or create a taxonomy for healthcare SOA services in the event that an existing bodyof-work is incomplete or insufficient. 118 Final Report HL7 Meeting—Atlanta, USA (May 2013) Existing terminologies, where applicable, will be utilised and/or extended. Where appropriate terminologies are not available, they will be developed to meet the needs of the use cases identified. PROJECT ACTIVITY/ISSUES AT THIS MEETING Significant progress has been made in this project since last WG meeting and as a result the project lead, Zoran Milosevic, has led the team towards ballot comment submission. The ballot reconciliation process was performed at this meeting. Several minor comments were accepted and the only major comment suggesting the development of a formal ontology to support reasoning and inferencing will require further clarification in the document, with the emphasis of this being a subject of a future release. It was also agreed to continue testing the service concepts in specific projects. There is currently interest from Canada Infoway to apply the concepts in the context of a new version of the Infoway Blueprint in terms of its business and systems architecture. It is anticipated that Infoway and Deontik experts will be leading this effort with the aim of reporting progress at the September 2013 meeting. 2.27.2.3 PROJECT (NEW): ORDERING SERVICES INTERFACE SPECIFICATION PROJECT PROJECT OBJECTIVE The VA has sponsored a project to develop a set of ordering service interfaces to support: • Delivery of alerts, recommendations and other notifications using email, SMS, VOIP and other communication channels • Tracking targets (e.g. relevant clinicians) to deliver the notification/alert messages • Standard ways to initiate ordering of pharmacy, lab, radiology, consult and nutritional services • Applications and service-to-service interactions, e.g. by CD • Subscription to clinical events of interest and receiving notice when new data is available The proposed Ordering Service is intended to complement existing SOA services and the SAIF Behavioural Framework (BF) for HL7. Pre-existing conceptual work in topics such as Composite Orders, Laboratory Orders, Nutrition, Medication, and FHIR Order and Prescription resources will be leveraged extensively to help document the necessary definitions, descriptions, graphics, and artefacts that are relevant. The Service Interface Specification will provide functional, semantic, and conformance profiles. PROJECT ACTIVITY/ISSUES AT THIS MEETING The primary sponsor of project is Orders and Observations (OO) and co-sponsored by Clinical Decision Support and SOA groups. It is targeting January 2014 for an initial informative ballot and DSTU ballot in May 2014. A project scope statement was presented at the joint OO-Patient Care-Clinical Decision Support WG meeting and approved at the joint meeting. 119 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.27.3 ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by SOA: Issue: The OMG MDMI standard can support many types of information mappings but is not sufficient to support general behavioural mapping. IT-014-09 Cross Platform Interoperability Implementation Guide for Immunisation SOA: SOA Service Ontology Action: Continue analysis of alternative approaches to support behavioural mapping, including the use of an MDMI standard and other MDHT components as well as new SOA Ontology concepts. Issue: The ontology paper included on the SOA ontology wiki page: http://hsspinfrastructure.wikispaces.com/HSSP+SOA+Service+Ontology provides the basis for the development of a rich set of discoverable and interactive e-Health services. When balloted it needs to be explained, discussed, socialised and commented upon by a wide audience within the health standards and software community. SOA Ontology WG HL7 Australia IT-014-09 NEHTA Action: Develop and present a brief paper for Standards Australia, outlining the approach and consider the application of SOA ontology for Australian e-Health, e.g. National Health Services Directory, the ELS and PCEHR. Note that the SOA Ontology is directly related to the e-Health Interoperability Framework, developed within IT-014-09, and thus this group is a natural home to progress this work. Action: Communicate SOA Ontology work to other relevant organisations and encourage the use of the ontology in SOA oriented solutions. SOA: Patient Care Services Co-ordination Project SOA/Patient Care: Ordering Services Interface Specification Issue: This seems to be a well-defined project and the only issue would be feasibility of executing it in sync with other projects of the SOA or other WGs. Action: Investigate how the PCC service can be expressed using the concepts proposed by the SOA Ontology project. The SOA/Patient Care ordering services interface specification project is relevant and of interest across the IT-014 and the wider eHealth standards community. Action: SOA/PC work on ordering services interface specifications to be reviewed by IT-014-06-04, IT-014-06-05 and IT-014-13 as basis for wider discussion and need for complementary Australian activity. IT-014-09 Future delegates to SOA Ontology WG IT-014-06-04 IT-014-06-05 IT-014-13 Australian eHealth communities 120 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.28 STRUCTURED DOCUMENTS 2.28.1 PROGRESS AT THIS MEETING Progress at this meeting is described in the context of the specific projects included in subsequent sections. A number of topics are at the level of the overall SD WG, including joint activities with other WGs, and these topics are described in this section. The meeting began with the committee providing an update of the SD WG 3 Year Plan. The intended topics for next 3 years are: • FHIR document resource monitoring. • C- CDA maintenance and update. • Quality (HQMF, QDM, QRDA and Handoff). • Patent Generated Documents. • Disease Specific. • HAI and Public Health Report. • CDA product Families – reflecting Product Family/Lines approach being developed by ARB. • International Version of C-CDA/IHE. • Template Repository. • Pilot of the IHE balloting process. • Implement recommendation of CDA publishing sub group. There was a joint session with the ARB where the ARB Co-Chair gave a presentation about product families and product lines which highlighted the need to establish separate governance, management and methodology groups (as defined in the HL7 SAIF) for the SD WG. Note that a governance group is already in place for FHIR project. The classification of CDA R2, CD R3 and C-CDA products into CDA Product Families/Product Lines is increasingly being socialising within the SD and this WG is an early adopter of the new product development approach being developed by ARB. There was a discussion between SD and Clinical Decision Support (CDS) about the need to consolidate various expression languages developed by different projects from these two WGs. For example, a concern was raised that different expression languages for expressing quality measures in HQMF and Health eDecisions (http://wiki.siframework.org/Health+eDecisions+Homepage) will present problems when linking systems that implement these specifications, e.g. if CDS systems need to intervene in cases where there are quality issues. The problem of non-alignment of these languages comes from the fact that they were developed by different groups, each with its own modelling approach: HQMF followed HL7's modelling structures to develop a representational model, which was then transformed into a XML representation through the HL7 predefined, automated process. The existing Health eDecisions work went straight to an XML representation which eliminated much of the implementation complexity implied by the HL7 automated process. That said, it was observed that there are the similarities between the two models that would help expose the commonality and it was agreed at the meeting that a consolidation across these languages is required. A proposed way forward is to define requirements for a common set of functionality for the languages and possibly use the SAIF framework to structure these requirements. It was noted that it would be helpful for meaningful use stage 3 if we use the same syntax for both HQMF and Gello. 121 Final Report HL7 Meeting—Atlanta, USA (May 2013) At the last WG meeting, a new ‘C-CDA Taskforce’ project was created to recommend to SDWG a process for managing and responding to implementer questions on C-CDA – as well as tooling to support it. The HL7 Wiki link for the C-CDA taskforce: http://wiki.hl7.org/index.php?title=Consolidated_CDA_Task_Force [Assessed 04, February 2013]. However, the project has not made it out of committee at this point due to the lack of a project facilitator role, and without a project facilitator, the project can’t move forward. At this point, the HL7 Board has deferred the question of moving forward with funding a Help desk (which is essentially what this project is about). The problem is that this project requires a major investment in time, and it is not possible to resource this on a volunteer basis. It remains to be seen when this project will be established. There was a joint session with Patient Care at which a new Project Scope Statement (PSS) was discussed for updates of C-CDA to better support care plans. Existing Consolidated CDA (C-CDA) needs to be enhanced by adding templates to represent priority data elements. Modified or new section level and document level templates are also needed for transitions of care and care plans, areas essential to patient care and the meaningful use of EHRs. Many organisations expressed interest in contributing to this project including: • ONC S&I Longitudinal Coordination of Care (LCC) WG; • Improving Massachusetts Post-Acute Care Transfers (IMPACT) Project; • New York eHealth Collaborative (NYeC); • Continuum of Care Improvement Through Information New York (CCITI); and • Center for Disease Control. The PSS was approved at this meeting and it was intended that the first specification is submitted for ballot in September 2013. In a joint session with FHIR, the FHIR document resource was presented and discussed. It was emphasised that FHIR focuses on what 80% of systems need (not 80% of use cases) and this also applies to the document resource. It is noted that FHIR will be submitted for DSTU ballot in September 2013 cycle, which is impressive considering that this new initiative started roughly a year ago. The ballot would coincide with CDA R3 which took substantially longer to reach this stage. It is also to be noted that FHIR will have all resources necessary to do C-CDA structures but will not be able to support extensions. It was also reported that Grahame Grieve is working on transformation of C-CDA to FHIR. Finally, a number of projects raised concerns about extending the C-CDA US realm focus into a more universal realm and this was recognised an important issue that needs to be addressed. Some C-CDA IG projects are already addressing this, including Patient Generated Documents, Forms and Questionnaire Response Documents and Care Plan C-CDA extensions and updates. 2.28.2 CURRENT PROJECTS The following projects are reported on in this section: • Project # 477: CDA Release 3. • Project # 900: Patient generated documents. • Project #853: IHE Health Story Consolidation, DSTU Release 1.1. • Project # 977: Questionnaire And Questionnaire Response Documents. • Project # 921: Clinical Oncology Data Standards. 122 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.28.2.1 PROJECT #477: CDA RELEASE 3 PROJECT OBJECTIVE The CDA R3 project is primarily involved in delivery of an updated release of the CDA standard: Clinical Document Architecture, Release 3 (CDA R3), including updated technical artefacts for the CDA RMIM, Hierarchical Description, and XML Schemas. Based on committee action it has been determined that CDA R3 will incorporate an updated Clinical Statement Model, utilize HL7 V3 data types, and R2 will be updated to be consistent with V3 Vocabulary model. PROJECT ACTIVITY/ISSUES AT THIS MEETING The CDA R3 meeting was primarily focused on addressing several remaining reconciliation issues and discussing preparation for editing and issuing a ballot in the September cycle. One issue discussed was how to handle weak semantics associated with the responsibleParty concept. The committee decided to deprecate the old pattern usage and align with the related modelling pattern used within Patient Administration. 2.28.2.2 PROJECT # 900: PATIENT GENERATED DOCUMENTS PROJECT OBJECTIVE In the ‘era of patient empowerment’, a specification for patient-generated clinical documents is seen to be required. Medical practices are looking for ways to allow patients to electronically complete certain tasks online such as filling out registration forms, health history forms, consenting to certain practice policies, and other types of clinical documents yet to be defined. As electronic document interchange increases, there is a growing need to communicate documents created by patients including those needed by providers and/or those document types defined by patients. Often, this is done through a secure web interface controlled by the patient such as a patient portal or a PHR. As more and more practices incorporate EMR technology into their practice workflow, they want to be able to import patient provided structured information into their EMR’s. This is being driven by the need to meet MU 2 requirements for patient engagement as well as other needs to reduce manual processes managing patient-provided data. The HL7 CDA Consolidation Documents types only address provider-initiated documents and do not incorporate guidance for patient-generated documents. It is necessary to create a new IG describing how to incorporate patient-generated input. In many cases, existing templates within the current Consolidation Template Library can be re-used with minimal modification. Additional information can be located at: http://wiki.hl7.org/index.php?title=Patient_Generated_Document_Informative_Document [Accessed 04, February 2013]. PROJECT ACTIVITY/ISSUES AT THIS MEETING The header part of the Patient Generated Documents IG was balloted at the previous WGM (Phoenix). This included header elements for US and universal realms. The purpose of this IG is to develop a standard way to evolve the health information ecosystem to capture records and make interoperable, patient generated information within the current framework of structured documents. The goal is to enable patients to participate and collaborate electronically with care team members. Ballot reconciliation is currently IN_PROGRESS: All 95 comments have been reconciled (April 19th) and the WG is now republishing with changes. The US realm PGD header will now be a further constraint on the C-CDA R2 header. The new IG will 123 Final Report HL7 Meeting—Atlanta, USA (May 2013) cite only further constraints on the C-CDA R2 header for the US realm. Further, the new IG will define a full set of constraints for the Universal realm, which will be generalizations of constraints in the US realm. The WG is planning to test use of the Trifolia tool to produce the IG for publication and will need help from the HL7 publication team and Trifolia Support in the publication process. They also stated a need to understand the process of the inclusion of the Patient Generated Document (PGD) header in the next version of C-CDA. Further, they need to encourage all other patient generated CDA documents to use the PGD header. Finally, they identified a need for the MU Phase 3 Patient Generated Health Data (PGHD) requirements to include the use of the HL7 PGD header. It is also to be noted that Australian requirements were adopted in this version for the header and that the project will be seeking input from the international organisations for future developments, such as the document body specification. 2.28.2.3 PROJECT #977: QUESTIONNAIRE AND QUESTIONNAIRE RESPONSE DOCUMENTS PROJECT OBJECTIVE This project will define a specification for the structured representation of a Form Definition and Questionnaire Response documents focusing initially on the requirements presented by the Continua Health Alliance Questionnaire Use Case. The specification will leverage HL7 CDA base standard and derived implementation guides. In the context of remotely monitoring patients, there is a need to facilitate the exchange of questionnaires between practitioners and patients. Electronic interchange of patients’ vital signs to the monitoring service or medical practitioner is but the first step in enabling effective dialogue and health practice. Of equal importance is the electronic interchange of meaningful questions and answers between the practitioner and the patient. The Continua Health Alliance is recognizing this need and has identified the following main areas for the meaningful exchange of the questions and answers: • Structured Form Definition Document representation. • Structured Questionnaire Response Document representation. The approach is to represent the Questionnaires and Responses in a structured document by reusing and/or enhancing existing CDA templates where possible while creating new CDA templates where necessary. The HL7 wiki for this project is available at: http://wiki.hl7.org/index.php?title=Form_Definition_and_Questionnaire_Response_Documents [Accessed 16, May 2013]. PROJECT ACTIVITY/ISSUES AT THIS MEETING At this meeting a number of reconciliation issues were addressed. The majority were minor issues, mostly to do with the cardinality of modelling elements. One major issue (and also negative vote by Keith Boone, GE) was that CDA was developed with the purpose of specifying clinical documents rather than the definition of document forms. Thus the CDA R2 is not the right vehicle to DEFINE forms. This is definitely an issue that needs to be addressed in CDA R3 but in the meantime it was agreed to address the problem by providing an extension in CDA R2 by including status code ‘New’ (suggested by Austin Kreisler and accepted by Keith Boone and the committee). This is a workaround and might not be entirely correct, but is probably the best that can be achieved in CDA R2. 124 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.28.2.4 PROJECT: HL7 IMPLEMENTATION GUIDE FOR CDA® RELEASE 2: IHE HEALTH STORY CONSOLIDATION, DSTU RELEASE 1.1 PROJECT OBJECTIVE The Consolidated Templated implementation guide contains a library of CDA templates, incorporating and harmonizing previous efforts from Health Level Seven (HL7), Integrating the Healthcare Enterprise (IHE), and Health Information Technology Standards Panel (HITSP). It represents harmonization of the HL7 Health Story guides, HITSP C32, related components of IHE Patient Care Coordination (IHE PCC), and Continuity of Care (CCD), and it includes all required CDA templates in Final Rules for Stage 1 Meaningful Use and 45 CFR Part 170 – Health Information Technology: Initial Set of Standards, Implementation Specifications, and Certification Criteria for Electronic Health Record Technology; Final Rule. It is commonly referred to as "Consolidated CDA" abbreviated C-CDA or CCDA. Further details can be found at: http://www.hl7.org/implement/standards/product_brief.cfm?product_id=258 [Accessed 16, May 2013] PROJECT ACTIVITY/ISSUES AT THIS MEETING At this meeting Keith Boone from GE raised three challenges that IHE has in harmonization with C-CDA. First, there is a problem with the existing template approach; in particular that template changes bubble up due to existing template methodology. The solution to this problem is to adopt template versioning in templates. This topic was to be further discussed at the Template WG. An interesting summary from the WG is provided in Keith Boone’s blog: http://motorcycleguy.blogspot.com.au/2013/05/versioning-templates-in-definitions-and.html [Accessed 18, May 2013]. The second problem is that C-CDA is US Realm but the IHE audience is International, which has particular implications for vocabulary. The WG solved this by identifying 97 concept domains that are used in their current template, i.e. replacing value set binding with concept domains. The third problem is scalability as a result of some 500 templates in the consolidated work. The WG solved this through automation using Model Driven Health Tools (MDHT), identifying a CCDA concept binding for problems. In his blog, Keith Boone, provides some insights and highlights the value of using MDHT tools: http://motorcycleguy.blogspot.com.au/2013/04/mdht-enables-ihe-pcc-and-hl7-ccda.html [Accessed 18, May 2013]. 2.28.2.5 PROJECT #921: CLINICAL ONCOLOGY DATA STANDARDS PROJECT OBJECTIVE This project will promote interoperability and information exchange among cancer care providers and patients. At this stage, focus is on immediate steps which will enrich the transfer of primary electronic information among providers and between providers and patients. It is an incremental step in establishing a rapid learning system and ultimately will benefit the research and reporting activities which, in turn, support the delivery of care. The Project will augment access to and reuse of this mission-critical information through development and promotion of a series of data specifications built on CDA templates. The initial work will develop templates required for exchange of the ASCO Treatment Plan and Summary and the disease-specific Breast Cancer Adjuvant Treatment Plan and Summary. These may be balloted together or incrementally. Later work will include developing templates which include the complete 125 Final Report HL7 Meeting—Atlanta, USA (May 2013) oncologic treatment history for a patient diagnosed with cancer. This may include diagnostic workup including laboratory, pathology, radiology, and molecular information; surgical treatment information; radiation treatment information; and chemotherapy/hormonal/immunotherapy treatments. In order to limit the initial scope, we will focus on the one disease scenario represented in the ASCO Breast Adjuvant Treatment Plan and Summary. This is moderately advanced stage breast cancer, i.e. breast cancer treated surgically with curative intent followed by adjuvant therapy (chemotherapy and/or radiation and/or hormones). This particular subgroup of cancer patients represents an ideal use case as most of the diagnostic and treatment approaches used in cancer are represented here. Further details can be found at: http://www.hl7.org/implement/standards/product_brief.cfm?product_id=258 [Accessed 17, May 2013]. PROJECT ACTIVITY/ISSUES AT THIS MEETING At this meeting ballot reconciliation was performed for the HL7 implementation Guide for CDA, Release2, Clinical Oncology Treatment Plan and Summary. This was considered a very good specification. Many of the minor ballot issues were addressed but a number of interesting issues are highlighted in the following text. There was a question about whether the LOINC code used should be specific (e.g. breast cancer) or more general (e.g. to catch broader cancer treatment plans). It was agreed that the current work by LOINC on document ontologies may provide a degree of freedom so there would be no need to note on the template ID that this is a specific breast cancer treatment, i.e. it can be a cancer treatment summary, and then rely on the external ontology to identify the specific document types and the related treatments. There was also an interesting observation by an oncology specialist which is of relevance for future modelling of cancer observations: In future, treatment may be based on genomics information because it is becoming increasingly evident that some cancer types (e.g. rare types of breast cancer) have the same type of mutation as other cancer types (e.g. ovarian cancer). It would be worthwhile to consider this likely development in current and future document design activities. 2.28.2.6 CONSOLIDATED CDA DSTU 2013 UPDATE (TO INCLUDE TEMPLATES SUPPORTING EXCHANGE OF CARE PLAN DOCUMENTS) This is an ONC funded project to update the current C-CDA DSTU to include a number of artefacts to support: • The incorporation of new data elements identified by ONC S&I LCC community providers for exchanging consultation notes, referral and transfer summary. • A care plan document using existing CDA templates and new templates identified by ONC S&I LCC community providers. • The linkage of consultation notes, referral and transfer summary to care plan documents where necessary and appropriate. • Alignment of the C-CDA care plan document with the Care Plan model currently being developed by the Patient Care project team. A project scope statement for this project was presented to the Patient Care and the Child Health work groups. Both groups voted to support this project and to become project co-sponsors. The plan is to have the relevant content ready for DSTU ballot in September 2013 and approval for publication in December 2013. 126 Final Report HL7 Meeting—Atlanta, USA (May 2013) The project timeline is very ambitious and is driven by the US Meaningful Use Stage 3 requirements. The three groups (ONC S&I LCC, Patient Care and Structured Documents) agreed to work closely together via weekly conference calls. 2.28.3 ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by Structured Documents: Issue: CDA R3 will be submitted for ballot in September 2013. NEHTA Action: Monitor new CDA R3 developments and understand industry acceptance and impact on Australian specifications that are based on R2. NEHTA and IT-014 committees to monitor the progress on this item. IT-014 CDA R3 HL7 Australia Also monitor interplay of R3 with FHIR specification that will be published in the same balloting period. Structured Documents: Patient Authored Documents Issue: The Patient Generated Document (PGD) IG is intended to provide implementation guidance on the CDA header and body elements for documents authored by patients or representatives of patients. The PGD header now includes international inputs based on Australian work to align better with international requirements. It is recommended that Australia be further engaged in the development of PGD body components. NEHTA IT-014 Action: NEHTA and Australia should participate and include Australian patient generated documents body elements to this CDAIG on PGD IG. This will enable Australian implementers and standards bodies to contribute and align with international standards for patient generated documents. Structured Documents: Issue: Electronic interchange of meaningful question and response documents between the practitioner and the patient. Questionnaire and Questionnaire Response CDA Implementation Guides Action: NEHTA and Australia should actively participate in the standardisation of Questionnaire and Questionnaire response documents. The Australian requirements for Consumer Entered Health Assessment and Bluebook Questionnaire requirements should be augmented to the universal realm. This is to align Australian consumer/healthcare provider questionnaire documents with the international questionnaire framework. Structured Documents: Issue: There is a need for formal methodology or best practices for developing CDA templates and implementation guides and appropriate tooling to support repeatable processes and deal with the proliferation of templates. Automation and open source tooling for clinical information modelling NEHTA IT-014 NEHTA Action: Investigate the use of Model Driven Health Tools (MDHT) as a way of providing programmatic access to the information model associated with templates and supporting a scalable and repeatable approach to the clinical information modelling. Consider positive experience reported at this WG meeting regarding harmonization between C-CDA and IHE and as a way of dealing with the proliferation of templates. 127 Final Report HL7 Meeting—Atlanta, USA (May 2013) Topic Issue/Action/Recommendations for Australia Recommended for Action by Structured Documents: Issue: The current C-CDA Implementation Guide specification is a good document from both the modelling and clinical perspective but is developed for the US Realm. IT-014-06-04 Clinical Oncology Treatment Plan and Summary Action: Consider this document for relevant future developments related to Australian e-health clinical oncology specifications. IT-014-06-06 IT-014-13 DoHA HL7 Australia Structured Documents: This project is relevant and of significant interest to IT-014-06-06, IT014-13 and many Australian jurisdiction initiating care plan projects. Australian eHealth communities Consolidated CDA update including Care Plan document 128 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.29 TEMPLATES 2.29.1 PROGRESS AT THIS MEETING There were some 14 delegates in attendance for the Templates WG session on the Friday morning including Richard Dixon Hughes and Zoran Milosevic from Australia. Topics discussed encompassed the following: • Formal representative to/from other WGs. Lisa Nelson is the (informal) representative for Templates WG in Structured Documents WG (SDWG); and the (formal) representative for SDWG in Templates WG and will ensure coordination between the two groups. At Lisa’s request, Mark Shafarman will help her do this. • Andy Stechisin remains the Tooling WG representative to Templates WG. • Project: HingX (Health Ingenuity Exchange) – Review use as a templates repository [see report under current projects below]. • Project #885: HL7 Templates ITS Pilot. Andy Stechisin reported [see current projects below]. • Templates DSTU R2 - including versioning, governance and Templates ITS. The next revision of the HL7v3 Templates specification was the main topic of discussion and drove the session into extra time [see report under current projects below]. Kia Heitmann gave an update on the ART-DECOR toolset, noting that: • It is being used on more than 12 projects in the EU. • The template editor is a draft version. • It manages versions and template relationships. • It has a repository of building blocks and references other projects: C-CDA, epSOS and IHE. • Further development is based on a Schematron Engine and includes support for open and closed templates, use cases and repositories. • In terms of follow-up actions, Kai Heitmann agreed to circulate an update on DECOR including information about the demonstration of an EKG report template, data on the “building block repository” of all CDA specifications and plans for a live demonstration. 2.29.2 CURRENT PROJECTS The following projects are reported on in this section: • Project: HingX (Health Ingenuity Exchange) – Review of use as a templates repository, related to Project #904: HL7/OHT Health Ingenuity Exchange Alpha Program Participation. • Project #885: HL7 Templates ITS Pilot. • Project: Templates DSTU R2 - including versioning, governance and Templates ITS, from archived project #272 Updated Template Interchange Format DSTU. 129 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.29.2.1 PROJECT: HINGX (HEALTH INGENUITY EXCHANGE) – REVIEW USE AS A TEMPLATES REPOSITORY PROJECT OBJECTIVE/ TOPIC SUMMARY HingX is a registry and repository for health related ICT resources. It was initially sponsored by the Rockefeller Foundation to enable an enterprise architecture approach to e-health for low resource countries they are also sponsoring. HL7 is committed to HingX as early adopter. Software versions are planned for release every one to two months. The HingX registry functionality has been tested against Templates Registry Business Process requirements. Also tested is the registration of artefacts produced by a number of HL7 WGs including Tooling, Publishing, SOA, and Templates. Metadata about these resources are stored in the registry and used to index and search for the resources. HingX has also defined a number of user types (e.g. individuals and groups such as project, team, organization, community) and user roles (to designate the set of actions that a user can perform regarding the resources related to a specific user group). It is possible that HingX can be a registry and repository for HL7 artefacts, which provides the following features: • A global resource registry with relationships between resources; • A reference repository; and • Access to other repositories. PROJECT ACTIVITY/ISSUES AT THIS MEETING There was limited discussion of HingX. Lisa Nelson (Cox Systems) reported that she is collecting information on all the extensions that have been created for CDA R2 including the numerous CCDA extensions. She is planning to review that collection for Templates registry metadata requirements and will share with Kai Heitmann and other interested parties and this will be posted on a wiki page linked to linked to the Structured Documents WG wiki. If time permits, and if the data and metadata analysis supports it, this could then be used to look at how HingX might be used to manage this type of information. [Subsequent to this discussion] Jane Curry volunteered to lead work with Kai Heitmann and Lisa Nelson on analyzing how HingX might be used as a potential templates registry. Again, if time permits, Templates WG as a group will also investigate using the now-published HingX API to test HingX as a possibility for a Templates Registry. This would include lessons learned from the several international groups now working with Templates (including CCDA, MDHT, Lantana, DECOR, NHS, NEHTA and Infoway), and be integrated with the findings of the Templates Registry Business Process Analysis document. 2.29.2.2 PROJECT #885: HL7 TEMPLATES ITS PILOT PROJECT OBJECTIVE/ TOPIC SUMMARY The focus of this project is to create an ITS that supports an interchange format in a canonical form for HL7 CDA templates. This project is a tightly focused sub-project of the templates interchange project (Project 130 Final Report HL7 Meeting—Atlanta, USA (May 2013) #272 in Project Insight) to move work forward in a specific area. The lessons learned from this project will feed back into the wider project. The requirements analysis for this project includes detailed review of requirements arising from MDHT (Model Driven Health Tools), Lantana Consulting Group’s Trifolia Workbench, DÉCOR, and Templates Registry Business Process Requirements. The Template ITS specifies templates using the German DECOR (data elements, codes, OIDs and Rules) approach to create HL7 CDA templates. It can be used to transform Trifolia CDA template data into a DECOR template (a 2-step conversion). Continuous gap analysis is being conducted on the transform and DECOR output layouts. It was previously reported (January 2013) on the basis of conversions done in late 2012 that DECOR appears to have been able to meet the requirements identified. PROJECT ACTIVITY/ISSUES AT THIS MEETING The project is continuing to progress, albeit slowly due to volunteer bandwidth issues. Andy Stechisin expects to have completed the conversion of the Lantana Trifolia templates to Templates ITS format (i.e. ART- DECOR XML) by the end of May. Keith Boone has contacts within MDHT and is starting to work with their templates; he will collaborate with Kai Heitmann on this and will contact MDHT representatives and request their participation in the Templates ITS project. The time is approaching to contact NHS (UK), NEHTA (Australia) and Infoway (Canada) to explore their potential participation in the Templates ITS project. 2.29.2.3 PROJECT: TEMPLATES DSTU R2 - INCLUDING VERSIONING, GOVERNANCE AND TEMPLATES ITS (FROM PROJECT #262) PROJECT OBJECTIVE/ TOPIC SUMMARY The “Templates DSTU”, HL7 Version 3 Standard: Specification and Use of Reuseable Information Constraint Templates, Release 1, was published in 2006 and expired under ANSI rules on 7 February 2010 DSTU. Despite now being expired, it is still the basis of templates that constrain v3 messages and, more significantly, CDA documents. Project #272: Updated Template Interchange Format DSTU was approved by TSC in August 2011. The aim of the project was to revise and republish the Templates DSTU to reflect the lessons learned from current approaches to defining and implementing templates, noting that: • There is an opportunity to align approaches and identify information needed to be able to communicate templates from one application to another. • At a minimum, the DSTU needs to provide guidance to designing, implementing and validating templates and the specification of a workable template interchange format. • While the primary focus was to be V3, application to V2 messaging was to be considered. • The work also aims to develop SAIF viewpoints at the three levels of: Business process level ((including public and private governance requirements and relationships among governance organizations; design level (addressing identification and versioning), and implementation (via repositories and ITS instances). • Template versioning, relationships and governance have been core issues needing to be resolved. 131 Final Report HL7 Meeting—Atlanta, USA (May 2013) A high level of consensus around these issues was considered a pre-requisite for a successful outcome from Project #262; however, the project became more complex with the advent of consolidated CDA, the proliferation of new families of profiles and templates (e.g. though IHE and national programs) and the creation of different tools to facilitate the application of these approaches. Particular stakeholders and interests include: The German dEcor project (Kai Heitmann), IHE profiles for CDA templates (Keith Boone), the Lantana Consulting Group’s Trifolia repository and templated C-CDA, the Templates ITS project and other stakeholders with interests in template exchange and repositories, including MDHT, US-DVA, and possibly UK-NHS and NEHTA. Failure to progress to development of a draft DSTU for ballot within the ANSI time limits meant that TSC was required to withdraw Project #262 entirely from the HL7/ANSI notified work program in December 2012 and since then the project has officially been archived. The work currently underway is therefore officially “preliminary” work to secure agreement on key issues – the approach to governance, design and characterisation of families of templates (i.e. issues of identification, versioning, publishing, and deprecation/retirement). When the project is ready to progress, it appears that a new PSS will be needed before the revised DSTU can be balloted. PROJECT ACTIVITY/ISSUES AT THIS MEETING Issues surrounding template versioning and relationships have been the subject of weekly Templates WG teleconferences from October 2012 to May 2013 (and are summarised in the report of the Australian delegation to the January 2013 WGM meeting in Phoenix). In terms of actually producing the draft Templates DSTU R2, the WG noted that the content needs to be separated into levels according to SAIF: • Governance level - the basic metadata that defines a family of templates (i.e. all the versions with a given template id) based on template registry requirements. • Design level – the information and computational processes for managing template version and publication status encompassing the needs of developers, registries and implementers. • Implementation level – the information and structures needed by implementers to know which version of a template should be used to create or validate a given instance, and if there are any particular requirements for later re-use of the data. All three levels must be consistent and applicable across any mix of closed/private and open/public contexts. The ways in which DECOR and IHE in particular use OIDs and other Template-IDs, versions and status metadata and creation/population dates of instances and, also, issues of backwards compatibility for existing templates and data were discussed at some length (with some reference to Trifolia and other contexts). The approaches used in each context are significantly different but it seemed that the various groups are prepared to work in a timely fashion toward an agreed common interchange format. More work is needed on use cases for versioning requirements at both the design and implementation levels, and their interactions. The Templates WG agreed that they needed to collect more use cases from the major implementers of templates identified above. There seemed to be general agreement that the draft templates DSTU R2 would encompass the following content: • Glossary. 132 Final Report HL7 Meeting—Atlanta, USA (May 2013) • The governance, design and implementation levels discussed above, including updated requirements for managing template versioning, relationships and instances. • Exchange formats and templates – the Templates ITS. • Conformance guidance (requiring liaison with the CGIT WG). • Contributions to harmonisation with IHE. Notwithstanding Project #262 having been withdrawn from the HL7/ANSI work program in December last year, Templates WG was still planning to have the project reinstated with a completed draft of the Templates DSTU R2 specification out to “for comment” ballot in the September 2013 ballot cycle. If this deadline is achieved, templates WG was hoping to get to hold the final DST R2 ballast for January 2014. These timelines seem highly ambitious. It is unclear how much of the final text is yet available and the project needs to be reactivated before it can be balloted. 2.29.3 ACTIONS FOR AUSTRALIA There are no further actions for Australia as a result of progress at this WGM; however, those actions put forward at the last WGM in January 2013 relating to monitoring of developments remain current 133 Final Report HL7 Meeting—Atlanta, USA (May 2013) 2.30 VOCABULARY 2.30.1 PROGRESS AT THIS MEETING The Vocabulary Working Group very much missed the expertise and guidance of Australian subject matter expert and Vocabulary Co-Chair Heather Grain at this working group meeting. Heather however was able to attend many of the meetings via teleconference from Australia. At this current working group meeting the three year plan for the group was updated with some projects moved to this area (project #499 Review ISO Principles and Guidelines of Terminology Maintenance and project #308 the Vocabulary conceptual model). General discussion amongst the work group included discussion of how the WG can be less reactive and increasingly approach work priorities in a less ad hoc fashion. From the Phoenix working group meeting the issue and recommendation of the use of HingX as a trial needing to be noted by Standards Australia, for consideration of adoption of the tool as: • It may be useful to Standards Australia as a platform for collaboration; and • The structure of resources needs to be well defined and input to the structure required. There was an action listed that further information would be provided at next HL7 meeting to support Standards Australia's review of tool and opportunities for use. This was not able to be followed up in the context of this meeting and will be added to the recommendations list to be followed up within IT-014 and the Vocabulary Work Group. 2.30.2 CURRENT PROJECTS The following projects are reported on in this section: • Project #948: Facilitator Training. • Project #947: Vocabulary Maintenance in the IHTSDO Workbench. • Project #945: Evaluate NLM vocabulary submission tool for HL7 use. • Project #324: Common Terminology Services Release 2 (CTS 2) – Normative (ballot next cycle). • Project #946: CTS2 incorporation of Shared Value Sets. • Project #874: V2 code table versioning and alignment to V3 vocabulary model. • Project #839: TermInfo – Implementation Guide for using terminologies in HL7 artefacts Release 2. • Project #806: Development of policies and procedures of an HL7 terminology authority. 2.30.2.1 PROJECT #948: FACILITATOR TRAINING PROJECT OBJECTIVE This project will create a series of webinars/podcasts and other forms of media to provide training to HL7 modelling, publishing and vocabulary facilitators. These will be offered as stand-alone tutorials that can be given on demand and on a scheduled basis to support new and existing HL7 Facilitators in understanding their role and how to perform related tasks. 134 Final Report HL7 Meeting—Atlanta, USA (May 2013) PROJECT ACTIVITY/ISSUES AT THIS MEETING Heather Grain presented a very comprehensive framework she has developed for Vocabulary facilitator training. This was presented to the working group meeting by Heather via teleconference. The progress for this will be to forward the completed framework and content to the Education Working Group for adoption. 2.30.2.2 PROJECT #947: VOCABULARY MAINTENANCE IN THE IHTSDO WORKBENCH PROJECT OBJECTIVE The scope of this project is to be able to represent the HL7 vocabularies, code systems, and value sets (content) in the IHTSDO Workbench to a degree that permits the IHTSDO Workbench to be used to perform vocabulary maintenance activities on that content. It will use the native vocabulary maintenance capabilities in the Workbench and identify what changes would be necessary to the Workbench to permit it to be used as HL7’s content development environment. A phased approach is recommended where Phase 1 builds upon the mapping previously completed between the Model Interchange Format (MIF) and the IHTSDO object model to complete a proof of concept based on Harmonization use cases. Phase 2 would only be required after successful completion of Phase 1. The estimate of effort required for Phase 2 could then be based on the required customizations identified in Phase 1. Phase 2 would require the ability to load (import) all the HL7 content from the MIF into a running instance of the IHTSDO Workbench as well as export content from the IHTSDO Workbench to a valid MIF representation. The import and export should be based on the current version of MIF 2.2.0. The phased approach is recommended to purposely separate the evaluation of functionality from larger development efforts. Thereby providing a means by which HL7 can complete the evaluation without committing to a larger development effort. PROJECT ACTIVITY/ISSUES AT THIS MEETING This project was reviewed and an action to look at establishing the next milestone date. 2.30.2.3 PROJECT #945: EVALUATE NLM VOCABULARY SUBMISSION TOOL FOR HL7 USE PROJECT OBJECTIVE The project will document the feasibility of implementing the NLM Request Submission System (USCRS) for use in Vocabulary submissions to the HL7 Harmonization process. It will define the functional requirements that the system must support as well as requirements to support the system from the perspective of both the technical components in the HL7 tooling environment and the process implications for harmonization and vocabulary maintenance processes. PROJECT ACTIVITY/ISSUES AT THIS MEETING This project is close to commencement and a Wiki page has been created under the Vocabulary Working Group on the HL7.org site. 2.30.2.4 PROJECT 324 COMMON TERMINOLOGY SERVICES RELEASE 2 PROJECT OBJECTIVE CTS2 is a model and specification for discovering, accessing, distributing and updated terminological resources on the internet. This community is a collection of individuals and organizations interested in 135 Final Report HL7 Meeting—Atlanta, USA (May 2013) developing, using, and consuming CTS2 resources. Structured terminologies provide a foundation for information interoperability by improving the effectiveness of information exchange. They provide a means for organizing information and serve to define the semantics of information using consistent and computable mechanisms. Terminologies are constructed to meet scope specific domain requirements. The domain specific nature of structured vocabularies often leads to variation in design patterns across the available terminology space. The ability to provide consistent representation and access to a broad set of terminologies enables multiple disparate terminology sources to be available to a community, and helps to ensure consistency across the domain space of that community. Service interfaces to structured terminologies should be flexible enough to accurately represent a wide variety of vocabularies and other lexically-based resources. The PIM specified in this document for CTS2 is intended to mediate among disparate terminology sources by providing a standard service information and computational model. The Information Model specifies the structural definition, attributes and associations of Resources common to structured terminologies such as Code Systems, Binding Domains and Value Sets. The Computational Model specifies the service descriptions and interfaces needed to access and maintain structured terminologies. Further information can be located at: http://informatics.mayo.edu/cts2/index.php/Main_Page [Accessed 11/02/13] PROJECT ACTIVITY/ISSUES AT THIS MEETING This is project was reportedly moving at a good pace with a recent normative ballot successful. The plan is that this project will be a normative standard with reconciled comments completed at the working group meeting. 2.30.2.5 PROJECT #946 CTS2 INCORPORATION OF SHARED VALUE SETS PROJECT OBJECTIVE The objective is to align data elements and align signature components. This work will also provide guidance and input on ‘impedance mismatches’. A demonstration of the Mayo Clinic value set service was provided. PROJECT ACTIVITY/ISSUES AT THIS MEETING An update for this project was presented and a website reference provided for value sets in the US realm https://vsac.nlm.nih.gov/ [Accessed 11/05/13]. 2.30.2.6 PROJECT #874: V2 CODE TABLE VERSIONING AND ALIGNMENT TO V3 VOCABULARY PROJECT OBJECTIVE The scope of this project is to review all code tables that have ever existed in the published HL7 V2.x standard and identify the type of V3 terminology model component (concept domain, value set, or code system) that is associated with each code table. If the V3 terminology model component is a value set or a code system, an Object Identifier (OID) will be assigned to them. Existing identifiers in V3 terminology model will be considered to leverage for re-use where possible. As a result of identifying the code tables, this will clearly outline which code tables are the code systems to constrain for value set implementation and identify the migration path to V3 terminology model. A constrain mechanism will be provided in a separate initiative. The analysis and action plan of the gap between V2 code tables and V3 terminology is out of scope of this project. Names for the new concept domains will be developed. A process for ongoing 136 Final Report HL7 Meeting—Atlanta, USA (May 2013) maintenance of V2.x (V2.9 and beyond) code tables and OID associated with each code table (as appropriate) will be established. End deliverable: All the code tables in chapter 2c will be augmented with the V3 model component determined by the project. Metadata for all the code tables will be augmented. An error list will be identified and given to the applicable WG to resolve. Appendix A will be regenerated from chapter 2c. PROJECT ACTIVITY/ISSUES AT THIS MEETING This project is now co-sponsored by the Vocabulary Work Group with primary sponsorship from the Conformance and Guidance for Implementation/Testing Work Group. 2.30.2.7 PROJECT #839: TERMINFO – IMPLEMENTATION GUIDE FOR USING TERMINOLOGIES IN HL7 ARTEFACTS RELEASE 2 PROJECT OBJECTIVE This project is to develop the policies and procedures for the establishment and ongoing operation of an HL7 Terminology Authority to administer terminology content in SNOMED CT. PROJECT ACTIVITY/ISSUES AT THIS MEETING Two TermInfo working sessions were conducted this WGM. Balloting as a normative standard is planned however it was acknowledged that a critical mass of interest was needed for this. The working group proposed to address issues of overlap between the terminology and information models. A lengthy discussion was held regarding issues of expression of negation in terminology and how this would be approached in CDA and Version 3. It was noted that there is no negation indicator in FHIR. A paper was proposed as useful for project direction. Also links with Modelling and Methodology were suggested. A template for use cases will be developed to support consistent information for different technology families. Moving forward with the negation issue the following was proposed by the working group: 1. Creation of a template using a fill in the blanks sentence approach for expressing requirements in concepts e.g., in family hx, allergies. 2. Make a start with the nine use cases in CDA. Possible development of a white paper with a succinct set of guidance to define when guidance will work for specific terminology families, when it is uncertain and when it will not work. Overall there was a requirement for deep understanding of terminological principles to actively participate in this project discussion. 2.30.2.8 PROJECT #806: DEVELOPMENT OF POLICIES AND PROCEDURES OF AN HL7 TERMINOLOGY AUTHORITY PROJECT OBJECTIVE This project is to develop the policies and procedures for the establishment and ongoing operation of an HL7 Terminology Authority to administer terminology content in SNOMED CT and define the responsibilities and relationships of HL7 to SNOMED CT and its use. The purpose of this group is to: • Manage the integration of SNOMED CT content into HL7 value sets, although other code systems will be under its purview. 137 Final Report HL7 Meeting—Atlanta, USA (May 2013) • Develop and manage a process and structure to triage and route terminology requests to IHTSDO or other external terminology organisations. • Define the processes to submit terminology to an external terminology body, and to define the scope of process within HL7. The need for this work was driven by the requirement to form a single point of contact with HL7 for IHTSDO content management. PROJECT ACTIVITY/ISSUES AT THIS MEETING The end date for this project was listed as the current working group meeting however no update or working session was presented in the context of this meeting. 2.30.3 ACTIONS FOR AUSTRALIA Topic Issue/Action/Recommendations for Australia Recommended for Action by Vocabulary: Issue: HL7 Vocabulary Working Group requires a deep understanding of the various technical vocabularies and project bases. At the current meeting the Vocabulary tutorial was cancelled and therefore mentored delegate was unable to fully engage in Vocabulary WG development. DoHA IT-014 Action: Consider Australian vocabulary priorities and potential for development and support of Australian subject matter experts in future delegations. Vocabulary: HingX Issue: The use of HingX as a trial needs to be noted by Standards Australia, for consideration of adoption of the tool as: Future HL7 3. review progress of 4. It may be useful to Standards Australia as a platform for collaboration; and The structure of resources needs to be well defined and input to the structure required. Delegation (to this project) Action: This action is carried over from a recommendation made by Heather Grain in the 2013 Phoenix Delegation Report. Please monitored by future delegations for information to support Standards Australia's review of tool and opportunities for use. 138 Final Report HL7 Meeting—Atlanta, USA (May 2013) 3. ACRONYM LIST Abbreviation Meaning ACCC Australian Competition and Consumer Commission ACMA Australian Communication and Media Authority ACSQHC Australian Commission on Safety and Quality in Health Care ACT Action ACTUG Australian Clinical Terminology Users Group ADL Archetype Definition Language AG Advisory Group AHIEC The Australian Health Informatics Education Council AHIMA American Health Information Management Association AHMAC Australian Health Ministers’ Advisory Council AHML Australian Healthcare Messaging Laboratory AIHW Australian Institute of Health and Welfare AIIA Australian Information Industry Association AMIA American Informatics Association AMT Australian Medicines Terminology ANSI American National Standards Institute ANZCTR Australia New Zealand Clinical Trials Registry API Application Programming Interface ArB Architecture Review Board AS HB Australian Handbook AS/NZS Australian/New Zealand Handbook AS/NZS ISO International Standards adopted by Australia and New Zealand ATNA Audit Trail and Node Authentication (IHE Standard) AU Australia abbreviation in the Int'l comment form AWI Approved Work Item 139 Final Report HL7 Meeting—Atlanta, USA (May 2013) Abbreviation Meaning BAM Business Architecture Model (HL7 ARB Project) BAU Business As Usual BRIDG Biomedical Research Integrated Domain Group. BRIDG is a collaborative effort of CDISC, the HL7 RCRIM WG, the (US) National Cancer Institute (NCI), and the US Food and Drug Administration (FDA) BRS Business Requirements Specification BYOD Bring Your Own Device Cal-X The California Exchange (Cal-X) is a data and information exchange to support healthcare, medical, public health and homeland security needs in a collaborative, shared, secure and cost-effective manner COPOLCO Consumer Policy Committee [ISO] CASCO Conformity Assessment CBCC Community Based Collaborative Care [HL7 Workgroup] CCD Continuity of Care Document CCS Care Coordination Service CCHIT (US) Certification Commission for Health Information Technology CD Committee Draft (third stage in developing an ISO or IEC standard) CDA Clinical Document Architecture CDC Centre for Disease Control and Prevention (within US/DHHS) CDISC Clinical Data Standards Interchange Consortium CDS Clinical Decision Support [HL7 Workgroup] CDV Committee Draft for Vote CEN European Committee for Standardization (Comité Européen de Normalisation) CENELEC European Committee for Electrotechnical Standardisation CEO Chief Executive Officer CGIT Conformance and Guidance for Implementation and Testing Committee CIC Clinical Interoperability Council [HL7 Workgroup] CIMI Clinical Information Modelling Initiative CIS Clinical Information Systems 140 Final Report HL7 Meeting—Atlanta, USA (May 2013) Abbreviation Meaning CMS Centres for Medicare and Medicaid Services (within US/DHHS) COAG Council of Australian Governments COM Comment conHIT2011 European Health Informatics Conference 2011 ContSys System of Concepts for Continuity of Care COR Corrigendum [to a Standard] CPOE Computerized Physician Order Entry or Computerized Provider Order Entry (In US) CRM Customer Relationship Management CTO Chief Technical Officer CTR&R Clinical Trials Registration and Results DAFF Department of Agriculture, Fisheries and Forestry DAM Domain Analysis Model (comprehensive model of a domain) [HL7] DAM Draft Amendment [Standards Australia] DCM Detailed Clinical Model DCOR Draft Corrigendum DEVCO Committee on Developing Country Matters [ISO] DHHS Department of Health and Human Services (US Government Agency) DICOM Digital Imaging and Communications in Medicine DIISR Department of Innovation, Industry, Science and Research DIS Draft International Standard (fourth stage in developing an ISO or IEC standard—the main opportunity for public input) DMP Dossier Médical Partagé (Shared Medical Record) (France) DoHA (Australian Government) Department of Health and Ageing DS4P Data Segmentation for Privacy DSTU Draft Standards for Trial Use (HL7 and ANSI) DTR Draft Technical Report [ISO and Standards Australia] DTS Draft Technical Specification [ISO and Standards Australia] DVA (Australian Government) Department of Veterans Affairs 141 Final Report HL7 Meeting—Atlanta, USA (May 2013) Abbreviation Meaning EC European Commission [the administrative arm of the EU] ECCF Enterprise Compliance and Conformance Framework EEC European Economic Community EFMI European Federation of Medical Informatics EHR Electronic Health Record EHR-FM EHR Functional Model EHRS or EHR-S Electronic Health Record System ELGA Austrian CDA Implementation Guide in Development ELS End Point Location Service EMEA European Medicines Agency EMR Electronic Medical Record or Electronic Medical Record System EN European Standard (Européen Norm) EPM Enterprise Project Management epSOS European Patients Smart Open Services—a European initiative (23 countries) to exchange pharmacy and EHR information, including prescriptions, across the EEC using IHE profiles and local standards (see http://www.epsos.eu) ETP Electronic Transfer of Prescriptions EU European Union EudraCT European Union Drug Regulating Authorities Clinical Trials FCD Final committee draft FDAM Final Draft Amendment FDIS Final Draft International Standard (for vote to publish) [ISO] FHIR Fast Health Interoperability Resources [HL7] FRS Functional Requirements Specification FYI For your information GCM Generic Component Model GDP Gross Domestic Product GP General Practitioner 142 Final Report HL7 Meeting—Atlanta, USA (May 2013) Abbreviation Meaning GSRN Global Service Relation Number GS1 An international SDO – primarily in the supply-chain domain GVP Good Pharmacovigilance Practices HCD Health Care Devices Committee HDF HL7 Development Framework HeD Health E-Decisions (project or group within ONC for decision support in MU3) HI Health Identifiers HIE Health Information Exchange HIMSS Healthcare Information and Management Systems Society HingX Health Ingenuity Exchange (HL7 International and OHT) HISC Health Informatics Standing Committee HITSP Health Information Technology Standards Panel HL7 Health Level Seven (International) HL7 ELC HL7 E-Learning Course HPI Healthcare Provider Identifier HPI-I Healthcare Provider Identifier for Individuals HPI-O Healthcare Provider Identifier for Providers HQMF Health Quality Measure Format HSSP Healthcare Services Specification Project [joint HL7/OMG] HTA HL7 Terminology Authority IC International Council (HL7) ICD10AM The Australian NCCH modification of ICD-10 code set for the coding of diseases and procedures ICD10-AM International Classification of Diseases, Version 10, Australian Modification ICD9CM International Classification of Diseases 9 Clinical Modification ICH International Conference on Harmonisation (of Technical Requirements for Registration of Pharmaceuticals for Human Use) ICHPPC International Classification of Health Problems in Primary Care 143 Final Report HL7 Meeting—Atlanta, USA (May 2013) Abbreviation Meaning ICNP International Classification for Nursing Practice ICPC2+ International Classification of Primary Care 2 ICSR Individual Case Safety Report [related to Medicines/Devices] ICT Information and Communications Technology IDMP Identification of Medicinal Products IEC International Electrotechnical Commission (an international SDO) IEEE Institute of Electrical and Electronic Engineers (US) (also an SDO) IG Implementation Guide IHE Integrating the Healthcare Enterprise IHI Individual Healthcare Identifier IHPA Independent Hospital Pricing Authority (Australian) IHTSDO International Health Terminology Standards Development Organisation IMATF International Membership and Affiliation Taskforce InM Infrastructure and Messaging [HL7 Workgroup] IP Intellectual Property IS International Standard ISO International Organization for Standardization ISO/CS ISO Central Secretariat ISO/TC 215 ISO Technical Committee (Health Informatics) IT Information Technology IT-014 Standards Australia Committee IT-014 (Health Informatics) ITS Implementable Technology Specifications ITTF ISO/IEC Information Technology Taskforce ITU-T International Telecommunications Union—Standards Division IXS Identity Cross Reference Service [OMG/HL7 HSSP] JI Joint Initiative on SDO Global Health Informatics Standardization JIC Joint Initiative Council (responsible for governance of the JI, with current members being ISO/TC215, CEN/TC251, HL7 International, CDISC, IHTSDO, GS1 and IHE) 144 Final Report HL7 Meeting—Atlanta, USA (May 2013) Abbreviation Meaning JSC-HIS Joint Standing Committee on Health Informatics Standards JSON Java script Object Notation JTC Joint Technical Committee JTC 1 ISO/IEC Joint Technical Committee 1—Information Technology JWG Joint Working Group KPI Key Performance Indicator LB Letter Ballot LIC Low Income Country LMIC Low and Medium Income Countries LOINC Logical Observation Identifiers Names and Codes [Regenstrief Institute] LPO Local PCEHR Officer MBS Medical Benefits Scheme MBUA Member Body User Administrators (person who maintain the ISO Global directory in each country) MDA Model Driven Architecture MDHT Model-Driven Health Tools MDMI Model Driven Message Interoperability MIC Medium Income Country MIF Model Interchange Format MLM Medical Logic Module MM Maturity Model MnM Modelling and Methodology [HL7 Workgroup] MOU Memorandum of Understanding MSIA Medical Software Industry Association MT Maintenance committee (IEC) MU2/MU3 Meaningful Use Stage 2 and Stage 3 (US Initiative) NASH National Authentication Service for Health NATA National Association of Testing Authorities 145 Final Report HL7 Meeting—Atlanta, USA (May 2013) Abbreviation Meaning NEHIPC National E-Health and Information Principal Committee NEHTA (Australian) National E-Health Transition Authority NH&MRC National Health and Medical Research Council NHCIOF National Health Chief Information Officer Forum NHIN (US) National Health Information Network NHISSC National Health Information Standards and Statistics Committee NHPA National Health Performance Authority (Australian) NHS (UK) National Health Service NIH (US) National Institutes of Health NIST National Institute of Standards and Technology (USA) NLM National Library of Medicine (USA) NMB National Member Body [of ISO or CEN] Normapme European Office of Crafts, Trades and Small and Medium sized Enterprises for Standardisation NP New Work Item Proposal (current ISO/IEC abbreviation) NPACC National Pathology Accreditation Advisory Council NPRM Notice of Proposed Rule Making (a step in the regulatory process in the US) NPC National Product Catalogue NQF National quality (measures) framework NSO National Standards Office NWIP New Work Item Proposal (obsolete ISO/IEC abbreviation—see NP) O&O Orders and Observations [HL7 Workgroup] OBPR Office of Best Practice Regulation OCL Object Constraint Language OHT Open Health Tools Foundation (www.openhealthtools.org) OID Object Identifier OMG Object Management Group ONC Office of the National Coordinator for Health Information Technology (within US 146 Final Report HL7 Meeting—Atlanta, USA (May 2013) Abbreviation Meaning Department of Health and Human Services) OSI Open Systems Interconnection OTF Organisation Taskforce [ISO TC 215] OWL Web Ontology Language PA Patient Administration [HL7 Workgroup] PACS Picture Archive and Communication System PAS Patient Administration System PASS Privacy Access and Security Service PBS Pharmaceutical Benefits Scheme PC Patient Care [HL7 Workgroup] PCEHR Personally Controlled Electronic Health Record PDAM Proposed Draft Amendment PDF Portable Document Format PDTR (Proposed) Draft Technical Report PGD Patient Generated Document [HL7] PHDSC Public Health Data Standards Consortium PHER Public Health and Emergency Response [HL7 Workgroup] PHR Personal Health Record PHTF Public Health Taskforce PIM Platform Independent Model PIP Practice Incentive Payment PIR Post Implementation Review PKI Public Key Infrastructure PM Project Manager PMBOK Project Management Body of Knowledge PMO Project Management Office PMP Project Management Plan 147 Final Report HL7 Meeting—Atlanta, USA (May 2013) Abbreviation Meaning PMS Practice Management System PMTL Project Management Team Leader PoC Point of Care PSM Platform Specific Model PSS Project Scope Statement [HL7] PSUR Periodic Safety Update Report PWG Pharmacy Working Group [HL7 Workgroup] QDM Quality Data Model of the US National Quality Forum RACGP Royal Australian College of General Practice RCPA Royal College of Pathologists Australia RCRIM Regulated Clinical Research Information Management [HL7 Workgroup] RDF Resource Description Framework (W3C) REST Representational State Transfer RFI Request for Information (a step in the regulatory process in the US) RFID Radio Frequency Identification RFP Request For Proposal(s) RHIO (US) Regional Health Information Organisation RIM Reference Information Model RIMBAA RIM Based Application Architecture RIS Radiology Information Systems RLUS Resource Locate Update Service (HSSP) RMES Records Management—Evidentiary Support (HL7) RMIM Refined Message Information Model RM-ODP Reference Model of Open Distributed Processing RO Responsible Officer SA Standards Australia SAIF Services Aware Interoperability Framework 148 Final Report HL7 Meeting—Atlanta, USA (May 2013) Abbreviation Meaning SAMHSA Substance Abuse and Mental Health Administration SC Subcommittee SD Structured Documents [HL7 Workgroup] SDO Standards Development Organisation SHARPS Strategic Healthcare IT Advanced Research Projects on Security SHIPPS Semantic Health Information Performance and Privacy Standard SIG Special Interest Group SKMT Standards Knowledge Management Tool SLA Service Level Agreement SMB Standards Management Board (IEC only) SME Subject Matter Expert SMTP Simple Mail Transfer Protocol SNOMED CT Systematised Nomenclature of Medicine—Clinical Terms SOA Service Oriented Architecture SOAP Simple Object Access Protocol SSD Single Sign On T3SD Technical and Support Services Steering Division TC Technical Committee TCM Traditional Chinese Medicine TCP/IP Transmission Control Protocol/Internet Protocol TEAM Traditional East Asian Medicine—this term, though inadequate, is used to represent Traditional Chinese Medicine, Traditional Korean Medicine and Traditional Japanese Medicine. TF Taskforce TM Traditional Medicine TMB Technical Management Board (ISO only) TOGAF The Open Group Architecture Framework TR Technical Report (an informative ISO or IEC standards publication) 149 Final Report HL7 Meeting—Atlanta, USA (May 2013) Abbreviation Meaning TS Technical Specification (a normative standards publication having a lower level of consensus than a full international standard) TSC HL7 Technical Steering Committee UAT User Acceptance Testing UCUM Unified Code for Units of Measure [Regenstrief Institute] UHI Unique Healthcare Identifier UML Unified Modelling Language UN United Nations VA Department of Veterans Affairs (US) VMR Virtual Medical Record VOC Vocabulary Committee [HL7 Workgroup] W3C World Wide Web Consortium WCM Web Content Management WD Working Draft (second stage in developing an ISO or IEC standard) WG Working Group or Work Group WGM Working Group Meeting WHO World Health Organization [UN Agency] WI Work Item WTO World Trade Organisation XDS (IHE’s) cross enterprise Data Sharing protocol XML Extensible Markup Language 150 Final Report HL7 Meeting—Atlanta, USA (May 2013) 4. 4.1 REPORTS FROM HL7 AFFILIATES AROUND THE WORLD HL7 ARGENTINA Website: http://www.hl7.org.ar. HL7 Argentina has pursued active engagement with health sector institutions, involvement in industry events and the continuing provision of educational services, including the following: • March 2013 – two-day HL7 Argentina Conference on at the University of Lujan in Buenos Aires focussing on v2 and CDA experiences • 19 April 2013 - full day workshop on standards for exchange of clinical information co-hosted with the nuclear medicine Association (FUESMEN) • 25-26 April 2013 - presentation to 4th International Conference on Hospital Management • July 2013 - participating in the "Expo Hospital" event in Santiago – Chile involving over 180 firms • August - HL7 Argentina paper being presented at MEDINFO 2013 in Copenhagen(August) • 16-20 September 2013 – jointly organising the 4th Argentinean Health Informatics Congress (CAIS 2013) “Connecting Healthcare Actors", with a full day of HL7 tutorials/workshop and coverage of V2-CDA-FHIR, QRDA (Quality Reporting Document Architecture), CDA around the world, and Introduction to IHE • 25-27 September – HL7 participation is planned in the main healthcare provider and services event in Argentina (www.expomedical.com.ar) including an information desk and HL7 workshop. • Health informatics conference to be held at Hospital Italiano de Buenos Aires - Planned presentation of pre-certification courses. certification testing, implementation experiences and presentations by invited international HL7 experts. • Joint activity with Universidad del Centro de la Provincia de Buenos Aires (UCPBA) – including delivery of 2-day course (Intro/ V2.x/CDA R2, 3 hours theory / 6 hours lab) with 50 undergraduate students taking optional 'Medical Informatics' stream and conduct of workshops under proposed MOU. Collaboration with the Health Ministry on interoperability projects has continued, including implementing a web service with XML and HL7 v2.5; and on the NOMIVAC (immunization); REDOS (blood bank); and REMEDIAR (medication) projects. As the creator of the HL7 Foundations e-learning course (ELC), HL7 Argentina continues to be strongly involved in both the development and delivery of the course, with the following highlights being noted: • Delivery and scalability of the course has been improved, with the current international edition in English having more than 300 students from 22 countries (including Australia) supported by 14 tutors from 9 countries. The current international edition in Spanish has over 50 students from 8 countries • Collaborative e-learning courses are being run with HL7 affiliates in Austria (finishes May 2013), Pakistan (started March) and Romania (started April); scholarships have been provided to students from Puerto Rico; and Australia is continuing its evaluation of HL7 e-learning materials • The first Portuguese edition of the ELC has been produced as a collaboration with HL7 Brazil • A FHIR unit is being developed for the course (with input from David Hay in New Zealand) The 2013 membership of HL7 Brazil remains stable at 24 and finances are also sound. 151 Final Report HL7 Meeting—Atlanta, USA (May 2013) 4.2 HL7 AUSTRALIA Membership is stable and there were several successful educational events held in 2012. The International HL7 Interoperability Conference (IHIC) is planned for October 2013 in Sydney, Australia. Affiliate input into this event is requested. 4.3 HL7 AUSTRIA No report was given at this WGM. 4.4 HL7 BRAZIL Website: http://www.hl7.org.br/ HL7 Brazil has been very active and works in close collaboration with other South American HL7 affiliates in HL7 Argentina, HL7 Uruguay, HL7 Colombia, HL7 Mexico, HL7 Venezuela and HL7 Chile, Translation of the HL7 e-learning course (ELC) into Portuguese is being carried out in conjunction with HL7 Argentina and Instituo Edumed and is largely complete. The first ELC in Portuguese is expected be run online from Sao Paulo commencing in August and will assist in spreading interest in HL7 and implementation skills among Portuguese-speaking countries including Portugal, Angola, Mozambique, Macau and Timor Leste amongst others. In 2012 HL7 Brazil also entered into a joint venture with the Brazilian health Informatics Association to share administrative facilities and to conduct joint educational activities. 3 courses have were conducted in the first semester - HL7v2, HL7v3 and Software Certification. More on the HL7 Brazil’s educational offerings is available on the HL7 Brazil education website: www.hl7.virtual.org.br. HL7 Brazil participates in the National Healthcare Standards Committee. By Ministerial Mandate 2073 executed in August 2011, the Brazilian government nominated the following standards for use in the national eHealth program: • HL7 for interoperability among systems for communicating laboratory orders and results • HL7 CDA as the architecture for clinical documents Other international standards and specifications approved that the same time included: • LOINC for codification of laboratory tests, • SNOMED CT for clinical terminology an • DICOM for image communication • openEHR reference model for defining EHR content • IHE PIXv3/PDQv3 profiles for access/update to the national unique identifiers database (currently with 220 Million people registered), supported by a PKI-based capabilities in a clinical ID card. The next national eHealth strategic planning cycle ran from August 2012 to May 2013 with a final strategic planning document being released on 30 April. The recommended strategies include: • Adoption of IHE profiles for IT infrastructure and PCC (Patient Care Coordination) • Hospital summary discharge and primary care continuity of care record to be the first CDA documents to be shared. 152 Final Report HL7 Meeting—Atlanta, USA (May 2013) HL7 Brazil publishes an HL7 newsletter, runs a local OID registry and has 5 working groups in the areas of: Nursing Informatics, Certification and Standards, Education, Telehealth and Healthcare Standards. Key partnerships include relationships with: • The Federal Medical Council (CFM), including a formal agreement establishing a joint program for certification of Electronic Health Record Systems. • Datasus - Department of Health Informatics and Information - Ministry of Health • ANS - National Agency for Supplementary Health • ABNT - ISO TC215 Mirror Committee, IMIA and the regional IMIA-LAC organisation Notable work on implementing HL7 in Brazil includes: • The national health identifiers register • Provision of an integrated pathology laboratory network in São Paulo City to serve a population of 20 million people. The network is based on 4 private and 2 public laboratories and uses CDA R2 for results, “green” CDA for orders and LOINC for lab test identification (the solution is deployed in 2 laboratories and is planned to be fully operational by year end). • laboratory integration for Belo Horizonte (in Minas Gerais State) - under development using CDA R2 for orders and results) The EHR system certification program being conducted jointly with the CFM is a major activity aimed at improving the quality of EHR systems, increasing information security, paperless record-keeping and helping physicians to select EHR systems for use in their practices. Establishment of the program included identifying requirements for EHR-S: security, structure, content and functionality and the means of testing and auditing these in real EHR-systems based on assessment involving suitably qualified technical opinion. Currently: 12 EHR systems have been certified; another 40 are being assessed; a new 2013 version of the best practice manual has been produced, international; and interest in the program has been expressed through ISO/TC 215 and requests for partnership with other health workers’ councils. More than 200 professionals have been trained and new auditors are being accredited. The next steps will be programs on the handling of Digital Medical ID and system/process maturity levels. HL7 Brazil has 20 members – 10 organisational members (all major corporations,, universities or health services) and 10 individual members (physicians consultants and IT professionals). HL7 Brazil looks forward to the possibility of hosting an HL7 working group meeting in 2016, or in 2017 after the Olympics in Rio de Janeiro. 4.5 HL7 BOSNIA AND HERZEGOVINA No report was given at this WGM. 4.6 HL7 CANADA The Canada Health Infoway Standards Collaborative (SC) is the singular standards organization that functions as HL7 Canada, IHE Canada, and the Canadian national member body for ISO/TC215 and IHTSDO A multi-tiered SC membership model was introduced May 2012 which has been very successful. As at the end of March 2013, there were 122 corporate members, 90 individual members and 15 student members subscribing as "premium members" (all are members of HL7 Canada). Overall, since the new model was 153 Final Report HL7 Meeting—Atlanta, USA (May 2013) introduced, there has been an 87% increase in membership, with numbers since the start of 2013 being stable. Some features of current and proposed HL7 activities in Canada include: • Tooling for HL7v3 implementers being available from Canada Health Infoway and being widely used by EMR vendors and others • Numerous CDA implementations underway within the provincial jurisdictions and a Pan-Canadian CDA Project is creating a standard CDA header and publishing a CDA Implementation Guide • Maintenance releases of Pan-Canadian standards planned for 2013 to address needs in the Public Health, Shared Health Records, Claims, Medication Management, Laboratory, Client Registry, Provider Registry, Location Registry, Record Access, Terminology and Infrastructure domains. These will support continued HL7v3 implementations underway across these domains, including: - Provider Registry – operating in multiple jurisdictions and supporting some 10K messages per month - Laboratory services and patient care (e.g., allergy) - Medication management, which is being implemented in multiple jurisdictions for exchange of HL7v3 messages for pharmacy dispense; integration with clinical Electronic Medical Record (EMR) systems for exchange of electronic prescriptions is underway. There are already more than 1 million HL7v3 messages per month being processed to support medication management. The SC also runs a range of training courses and activities including : Orientation to HL7 v3 (46 recent participants), Foundations of HL7 v3 for Technical Professionals (11), Orientation to CDA (68), Introduction to HL7 v2.x (7), Introduction to HL7 v3 (11), customised HL7v3 workshop (14 and Introduction to CD (8 participants). 12 candidates successfully completed HL7 Certification 4.7 HL7 CHINA No report was given at this WGM. 4.8 HL7 COLOMBIA No report was given at this WGM. 4.9 HL7 CROATIA No report was given at this WGM. 4.10 HL7 CZECH REPUBLIC No report was given at this WGM. 4.11 HL7 FRANCE HL7 France is part of the "Interop Sante" collaborative which is also home for IHE France. The main French interest in HL7 relates to the use of CDA in the two major national programs: • DP (Dossier Pharmaceutique or medication record) uses CDA for the electronic transfer of prescription and dispense record documents. The DP program is relatively successful with information on some 26.18 million persons being held at the end of April 2013. 22,220 or 97.6% of pharmacies are participating. 154 Final Report HL7 Meeting—Atlanta, USA (May 2013) • The DMP (Dossier Medical Partagé or shared medical record) is being taken up at a much slower rate. At the end of April there were some 329,000 DMP records nation-wide, with some 60,000 in the Picardy region, which has the highest per capita uptake. HL7 France will be hosting the May 2015 WGM in the vicinity of Paris on behalf of the European affiliates (subject to final arrangements for organisation being resolved). 4.12 HL7 GERMANY There is continued collaboration with other German-speaking countries in the region focussed on: • Understanding, responding to and managing risks arising from the changes in HL7 International HL7 IP policy and its impact on affiliates in the region. • A shared HL7 journal published jointly in German by the HL7 affiliates in Germany, Switzerland, Austria and Luxembourg. • Expanding use of ART-DECOR tooling for managing the production and maintenance of templates, value sets and other HL7 modeling artefacts, particularly for Germany and Austria but extending to some other European countries including The Netherlands and Norway (with more to come). This product set is being developed as the core of a tooling strategy to support regional implementations. • The eHealth Interoperability Forum which was run collaboratively with IHE Germany in 2012 will be run again as an HL7+IHE Annual Plenary Meeting in October 2013 in Göttingen with the aim of expanding collaboration nationally and internationally [unfortunately this clashes with ISO/TC 215 in Sydney]. This is an incremental step in the direction of a potential standards collaborative. • Enhancing and updating the content of the Wiki on national and international standards and their use. This is to support marketing and dissemination of information is on HL7 standards and their use. With contributions from across the continent, HL7 Germany has led production of the third edition of the HL7 Europe Newsletter. This is available online at: http://www.hl7.eu/download/feun-03-2013-web.pdf. It is also available as marketing collateral for European HL7 affiliates with printed copies being distribution at the eHealth week in Dublin (in May) and planned for Medinfo in August in Copenhagen. Domestically, recent highlights have included: • Expanding collaboration with IHE Germany and other parties, like gematik (infrastructure). • A growing role for HL7 Germany supporting the development of national e-health infrastructure; several more implementation guides have been produced to support: - Discharge Letter (Release 2), Infectious Disease Reporting and Cancer Registry Reporting. • HL7 Germany providing comments to the German Government on a strategic study by the Health Ministry on Interoperability of health information. • Participation in a large health IT vendor exhibition - conhIT in April in Berlin. • An upcoming retreat planned for the HL7 Germany Board of Directors to discuss strategic initiatives, particularly any new initiatives in response to the free licensing of HL7 standards. • Making contributions at HL7 International on behalf of the German membership and feedback on the activities of HL7 task forces and key Working Groups (CGIT, Security, Templates). Despite the feared fall out from free licensing of HL7 standards, membership has been stable, still slightly increasing, with considerable focus on HL7 Germany being more widely relevant to local and regional needs. 155 Final Report HL7 Meeting—Atlanta, USA (May 2013) 4.13 HL7 INDIA The international Council welcomed Dr Lavanian Dorairaj MBBS, MD as the recently elected Chair of HL7 India and noted the contribution of his predecessor Prof Supten. Dr Dorairaj summarised activities in the previous year, the current year of the steps being taken to provide value for members. Membership growth has been relatively slow and currently stands at 11 organisations and 26 individuals. Nevertheless interest in eLearning courses (ELC) has been strong with two coursed having been run in the last year. Of 286 candidates undertaking the course 198 passed. There has been increasing positive interaction with the Ministry of Health and Family Welfare of the Government of India (MH&FW-GOI) and the Centre for Health Informatics (CHI) as the coordinating centre for e-Health activities in India. The annual General Body Meeting (GBM) took place in April 2013 and with a refreshment of the Board was an opportunity to discuss consider past performance under way forward, particularly with respect to the changes brought about by the ability to download HL7 International standards free of charge. New directions being pursued include:- reaching out proactively to industry, universities and hospitals, moving to hire a marketing agency/person, updating the HL7 India web presence and membership tracking. A stronger value proposition is required to attract and hold members, with the following initiatives being under consideration: • Setting up a service to test and certify e-health applications (something like CCHIT in the US), and publicising the results of positive certification • Supporting HL7 implementation by providing strategic advice and support and (still under consideration) “easy connect widgets” • Providing member rebates for training and HL7 activities • Working with government to recognize HL7 as the standard for healthcare Interoperability, and working closely with IHE India, formed in April with HL7 India being a member. APAMI 2014 is being held in New Delhi, India and will provide potential opportunities for wider engagement. 4.14 HL7 ITALY Web Site: http://www.hl7italia.it/. Chair: Stefano Lotti, CTO : Giorgio Cangioli. HL7 Italy is carefully monitoring its membership renewals in light of the recent change to license HL7 International standards and other selected intellectual property (IP) free of charge. Although membership subscriptions have yet to close, it appears that with 38 organisational members (compared with 43 in 2012) and 13 individuals having renewed, a small reduction in membership is possible but it is unclear whether any drop-off is due to the change in IP policy or to the current economic crisis. The reduction appears to be mainly in small enterprise; almost all of the leading Italian Health IT companies have remained as members of HL7 Italy for 2013. A key initiative involving HL7 Italy is the proposed creation of an EHR System Functional Model for Italian Regions addressing the new legal framework for EHR in Italy and supporting uniform core features in EHRs for use across the Italian regions. An initial conference was held on 28 January in Bologna hosted by EmiliaRomagna Regional Administration attracting more than 75 attendees with very positive feedback. The conference discussed issues and challenges of a cohesive Italian EHR development and increased understanding of the relevance of tool, such as the HL7 EHR-S FM to homogenize and govern the 156 Final Report HL7 Meeting—Atlanta, USA (May 2013) development of interoperable EHR systems. Speakers came from several regional administrations, interregional consortiums and from research institutions. As a follow up, HL7 Italy and CISIS (IT interregional PA consortium), have established a working group on Italian EHR functional model (FSE-FM) with membership from 11 Regional Administrations/Agencies, CNRICAR (National Council of Research), CISIS, and HL7 Italy. Work started in February, with initial drafts of scenario documents being released by regions in subsequent meetings. The first HL7 Italy eLearning: course (ELC) was successfully completed (thanks to Fernando Campos and Diego Kaminker of HL7 Argentina). The next course will be scheduled for September 2013. On 23 February HL7 Italy held a kick-off meeting in Rome to start a project to produce a SOA HSSP IG (no further detail was provided). HL7 Italy was also supporting the “Summit on Evolving an Open e-Health Platform. Bringing Down Business, Policy, Information, and Technology Barriers”, which was held in Berlin on 17 June. The summit sought to combine selected presentations and case studies with community dialogue to chart a course for e-Health that is practical and implementable. Case studies were presented from Victoria Health (Australia), the Mayo Clinic (US), the National Health Service (UK), Phast (France) and others and addressed topics ranging from information representation to architectural “services” to business policies. 4.15 HL7 JAPAN Ken Toyoda, Director & Secretary General, HL7 Japan, presented the report from HL7 Japan. HL7 Japan has a strong membership base which, as at the end of 2012, included 343 industry members that are also members of JAHIS (Japan Association for Health Information Systems), 24 other industry members, 8 user organisations (hospitals/clinics) and 98 individual members, giving a grand total of 473. HL7 Japan’s plans for 2013 include: • Developing a “Discharge Summary Standard” based on Consolidated CDA templates • Delivering a full version of the e-Learning course in Japanese - with a pilot session having been successful • Producing a Japanese "Guide to HL7" based on translation of material on HL7 messaging • Establishing an HL7 conformance test capability following successful completion of trial testing • Continued support of HL7 Asia, particularly through organisation of the first HL7 Asia symposium to be held in Tokyo in July and also production of the HL7 Asia News etc. 4.16 HL7 KOREA No report was given at this WGM. 4.17 HL7 LUXEMBOURG No report was given at this WGM. 4.18 HL7 NEW ZEALAND A presentation from HL7 New Zealand was not given in the general session but it was the turn of Dr David Hay, Chair of HL7 New Zealand, to give a fuller presentation to the affiliate chairs meeting in the “political realities” segment. As reported in the section of this report on the International Council (Affiliate Chairs) 157 Final Report HL7 Meeting—Atlanta, USA (May 2013) meeting, he outlined the current organisation of e-health activity in New Zealand, current national strategies and the role played by the various government bodies, HL7 New Zealand and other groups. 4.19 HL7 NETHERLANDS Roel Barelds, TSC Co-Chair and HL7 Netherlands board member presented the HL7 Netherlands (HL7 NL) report as proxy for the Chair, Robert Stegwee. Website: www.hl7.nl HL7 NL only has organizational members. The total membership of 215 is split evenly between vendors/consultancy firms (108) and healthcare/institutional members (107). These members are represented by 556 individual persons registered with HL7 NL. Membership is currently stable. HL7 NL is restructuring the organisation of its technical activities from working groups to project teams to increase active member participation and achieve more focus within individual projects. The business/revenue and membership models are also under review. The potential consequences of the change in HL7 International's IP policy continue to be monitored. From the strategic perspective, HL7 NL entered into a formal agreement with IHE-NL on January 1, 2013 with the aims of: • Delivering combined HL7 implementation guides and IHE profiles: “packaging” • Setting joint priorities and conducting joint public relations, marketing and training • Speaking with a single voice on all national standards policy levels HL7 NL will also be part of a wider collaboration amongst all the major Dutch health Informatics standards development and implementation organisations under a charter executed on 27 May by HL7 NL, IHE-NL, Nictiz, NEN and the Dutch IHTSDO National Release Centre (NRC). The objectives of this collaboration are to improve nationwide coordination of health informatics standards activities; an end to scattered initiatives; and the conduct of joint projects. More information is available in the third HL7 Europe newsletter available from: www.HL7.eu. Recent and planned HL7 NL activities include: • Publication of an updated HL7 NL v2.4 implementation guide (in December 2012) • Definition of a single universal generic document for nationwide transfer of clinical documents to support continuity of care - supported by a Dutch CDA Implementation Guide based on CCD/CCR • FHIR presentations and publications (by Ewout Kramer) • Completion of 30 DCM models - an area of on-going work • A very successful series of training programs (led by Rene Spronk) - with classes on HL7, IHE and DICOM • The HL7 Jubilee Conference in December 2012 celebrating 20 years of HL7 Netherlands, the first HL7 affiliate • Joint Health Architecture Conference with IHE, Nictiz and NAF on June 21, 2013, and • MIC 2013 - the Annual Medical Informatics Conference, planned for November 2013. 4.20 HL7 NORWAY Line Andreassen Saele presented a report from HL7 Norway. As one of the most recent affiliates to be established, the principal activity is running HL7 information days in Norwegian. One information day had been conducted in 2013, with others planned for different locations around the country in June and after 158 Final Report HL7 Meeting—Atlanta, USA (May 2013) the summer break. Some training has been delivered in recent years with the assistance of experts from other Northern European affiliates. Training on IHE is planned for June and on FHIR in September. 4.21 HL7 PUERTO RICO The report from HL7 Puerto Rico (HL7 PR) was given by Julio Cajigas, Chairman HL7 PR. As one of the most recently established affiliates, HL7 PR has been most active and is operating on a firm professional basis, achieving recognition as a partner in the development and realisation of the national e-health strategy. HL7 PR has 5 office-bearers - Chairman, Secretary, Treasurer, Membership Coordinator and Workgroup Coordinator. The membership structure has 6 categories of member loosely mirroring relevant HL7 International membership categories. The categories and associated annual fees are: – individual member $525, organisation $910, supporter $1,400, medical group $805, student $50 and health professional $100. The fee-scale for the main categories is 70% of the corresponding base level fee for the corresponding HL7 International membership categories. No indication was given of member numbers. HL7 PR activities are operating to an aggressive timeline at a time of reform in the use of electronic information in the Puerto Rico health system. Milestones along this pathway have included: • Participation in the 2012 and January 2013 WGMs • Hosting a two-day Caribbean HL7 Conference in February, 2013 - keynote speakers included Ed Hammond (Secretary and Chair Emeritus of HL7 international), Philip Scott (HL7 UK and Co-chair HL7 International Council) and Diego Kaminker (HL7 Argentina and HL7 Board Member). • Conducting online education through participation of Puerto Rican students in the HL7 e-learning course (ELC) – including negotiating scholarships to increase participation • Planning to hold an HL7 Educational Summit in Puerto Rico in August 2013. HL7 PR has also been collaborating with the Government (Health Department and economic development), the clinical professions, health care facilities, industry/trade associations and others as a participant in the I4HIE ("Initiative for Health Information Exchange"), which is developing and implementing a federated collection of interconnected health information exchanges (HIEs) that will evolve into a nation-wide health information network. Early experiences with the electronic transfer of prescriptions demonstrated the importance of building solutions to rigorous national e-health standards. The initial (Phase 0) pilot of I4HIE is operating in the West of the country supporting a population of some 580,000 people and 7 hospitals. It is being extended incrementally, and will shortly include another 90,000 people and another hospital. In the pilot stage, HL7 CDA is being used for a CCD Discharge Summary. Ultimately The Puerto Rico health information exchange envisages a federation of local HIE engines, each supporting a hospital regional health information organisation supporting exchange of CDA-based clinical documents between hospitals, patients, physicians, consultants, pharmacies, insurers, public health agencies and HIE engines in other regions, as well as the exchange of message-based laboratory communications. HL7 has a series of meetings to conclude legal formalities and secure its participation in upcoming stages of the I4HIE program and its involvement has support from the Health Department and PRTEC (PR TechnoEconomic Corridor). 159 Final Report HL7 Meeting—Atlanta, USA (May 2013) Puerto Rico is engaged in a health reform program being led by Dr Jorge Sánchez. Future directions include development and collection of locally agreed metrics for meaningful use and further expansion if the I4HIE program. A key national objective is to be a Caribbean leader in eHealth interconnectivity to support two-way trade in health services with facilities in the US and across the Caribbean. HL7 Puerto Rico offers itself as a manageable case study for implementation of HL7 in support of widespread health information exchange and a potential host for an upcoming HL7WGM 4.22 HL7 PAKISTAN No report was given at this WGM. 4.23 HL7 SINGAPORE No report was given at this WGM. 4.24 HL7 SPAIN No report was given at this WGM. 4.25 HL7 SWITZERLAND The HL7 Switzerland (HL7 CH)report was presented by,Beat Heggli, Chair HL7 CH, who noted that there had been no changes in membership since last meeting, notwithstanding the changes in HL7 International's IP policy allowing its standards to be licensed free-of-charge. Following recent HL7 CH elections, there have been some changes on the Board with Marco Demarmels being the Chair-elect. HL7 CH is developing a CDA Implementation Guide for reporting laboratory-results to public health authorities as a joint venture with IHE-CH. This is proceeding in stages. The first stage addresses notifiable results reporting and is nearly finished for approval by government. The second stage will produce a CDA implementation guide and Schematron rules for laboratory results used in organ transplantation. For use in Switzerland, it is also likely these documents will need to be translated into French and Italian, which may be of interest to some other affiliates. 4.26 HL7 TAIWAN No report was given at this WGM. 4.27 HL7 UK Dr Philip Scott, Chair HL7 UK presented the HL7 UK report. Website: www.hl7.org.uk Total membership remains unchanged at 162 members although there has been a shift from organisational membership to personal membership. “Free IP” has not yet had any discernible impact on membership, but general awareness is still low and many uncertainties remain. Recent and planned HL7 UK activities include: • A FHIR meeting led by Ewout Kramer 160 Final Report HL7 Meeting—Atlanta, USA (May 2013) • CDA Forum webinars run by DHID/HSCIC [Note: the Department of Health Information Directorate (DHID) has been disbanded and some of its functions have been taken over by the Health and Social Care Information Centre (HSCIC)]. • Planning underway for the June AGM and associated technical committee meeting • Training courses on HL7v2 and UK Interoperability Toolkit (ITK) • Academic outreach: 2-day Summer School in July • Increasing collaboration with IHE-UK, the BSI IST/35 Health informatics committee, BCS Health SIG. New types of activities and approaches have been emerging from the UK government in recent times. The previous “Information Sharing Challenge Fund” (ISCF) activity run by the former DHID was noted. This attracted 95 applications seeking £5.6m for local NHS interoperability projects to be completed by Q1 2013. 43 projects involving 40 companies, most of whom were unfamiliar with the ITK, were awarded funding of £2.17m. 23 projects were fully delivered, 12 were partly delivered and 8 did not deliver. ISCF has demonstrated the feasibility of rapid CDA solution development/deployment by vendors new to the market, given tight NHS specification and implementation support. A further round of competition has now been announced, with an additional round if KPIs are achieved. The latest NHS re-structuring still settling down. The commissioning power now rests with “NHS England” and the residual CFH roles of DHID have now been taken over by the HSCIC. Its website is at: www.hscic.gov.uk/systems. The UK Government is making a major investment in EHR based research collaborations with four regional consortia being funded (university/NHS/industry). They need to interoperate but academic projects often use theoretical or home-made rather than real-world standards. There is a substantial opportunity to embed re-usable standards and engage a new stakeholder group. HL7 being a truly open standard is a critical success factor in realising this opportunity. The HL7 UK Management Board is developing new value streams with emphasis on implementation support. There are opportunities for HL7 UK to leverage and facilitate interchange of technology and approaches used in ISCF work but only if concern over HL7 International’s IP licensing policy in relation to derivative works can be resolved satisfactorily. These opportunities are being held back because UK Government is increasingly looking to support "open" standards and stated limitations on creation and distribution of derivative works in the HL7 International licence for its “free IP” do not look like an ‘Open Standard’ to HL7 UK or NHS England - hence the need for further discussion of these issues, which is now taking place. Other planned engagement and activities included participation in EU eHealth Week in Dublin, May 2013 (hosting the fully-booked free HL7 session on Monday afternoon) and hosting a meeting of European affiliates on Tuesday morning. 4.28 HL7 US The report of the US representative to the international Council was presented by the Chair of HL7 International, Dr Don Mon, as a proxy for Dr Ed Hammond, who could not be present at the General Session. As all US activity takes place within HL7 international, rather than through a separate affiliate, Dr Mon's presentation focused on how HL7 International activities in the US realm support US national initiatives. 161 Final Report HL7 Meeting—Atlanta, USA (May 2013) HL7 responded to the Office of the National Coordinator for Health IT (ONC) and the Centers for Medicare and Medicaid Services (CMS) Request for Information (RFI) on Advancing Interoperability and Health Information Exchange (Interoperability RFI), identifying the needs for: • a data sharing culture and infrastructure. • use of the HL7 Health Quality Measure Format (HQMF) standard format for documenting the content and structure of quality measures • Adoption of Healthcare, Community Services and Provider Directory standards • Vocabularies, and coordination of -Blue Button with C-CDA, The CMS Continuity Assessment Record and Evaluation (CARE) project is using CDA specifications for exchanging standardized patient assessment data, HQMF and QRDA The Centers for Disease Control & Prevention (CDC)/National Center for Health Statistics Public Health Reporting Initiative (PHRI) are engaging with HL7 on the development of the EHR-S FM Public Health Functional Profile. Within the ONC S&I (Standards and Infrastructure) Framework there are ten active areas in which HL7 volunteers are engaged, for example: • Health eDecision: HL7 CQI (vMR/QDM harmonization) • Electronic submission of medical documentation (esMD): structured data, records management & evidentiary support • Transitions of Care, Longitudinal Coordination of Care: HQMF, QRDA HL7 is a participant in the Structured Data Capture initiative, which has scope: “To define the necessary requirements (including metadata) that will drive the identification and harmonization of standards to facilitate the collection of supplemental EHR-derived data.” This involves the development and validation of a standards-based data architecture so that a structured set of data can be accessed from EHRs and be stored for merger with comparable data for other relevant purposes to include: electronic Case Report Form (clinical research, including Patient Centered Outcomes Research Initiative), Incident Report (patient safety leveraging AHRQ common formats), Surveillance Case Report Form (public health reporting of infectious diseases). It will result in four standards • A standard for the Common Data Elements that will be used to fill the specified forms or templates • A standard for the structure or design of the form or template (container) • A standard for how EHRs interact with the form or template • A standard to enable these forms or templates to auto-populate with data extracted from the existing EHR. 162