Green Synthesis of Camphor: Lab Report Analysis
advertisement

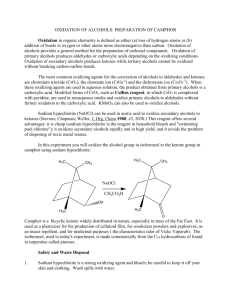
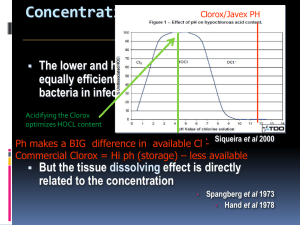
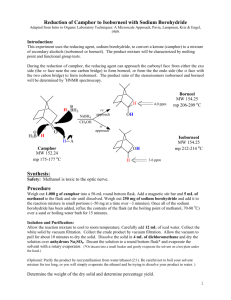
A Green Synthesis of Camphor Introduction: The objective of the experiment is to oxidize a secondary alcohol, isoborneol to ketone camphor and to determine whether the purity is at least 95%. Secondary alcohols can be converted to ketones using very strong oxidizing agents such as chromic acid. However, there exists chromium compounds that are known to be corrosive and generally harmful and pose as a legal disposal issue due to their harmful nature. In this experiment camphor will be prepared by oxidizing a secondary alcohol, isoborneol. An environmentally friendly way to oxidize a secondary alcohol is by using bleach which contains 5.25% of sodium hypochlorite. Adding small amounts of acetic acid facilitates the reaction by converting sodium hypochlorite to hypochlorous acid (HOCl). This is the active oxidizing agent. Oxidation of isoborneol is an exothermic reaction, in order to avoid the creation of side product; the temperature should be controlled below 40 degrees Celsius. Extreme heat can lead to creation of camphoric acid, so heat should be controlled by using the ice-bath as needed. Enough sodium hypochlorite should be added to ensure that reaction has reached its completion. When hypochlorous acid (HOCl) is present in excess, a small portion can be transferred to starch and potassium iodide indicator paper and tested. The indicator paper should turn dark blue. Starch and potassium iodide will oxidize iodide ions to iodine. Excess HOCl that remains after can be destroyed by sodium bisulfite (reducing agent). Camphor is very compact and its molecular structure is symmetrical which gives it the property to change directly from a solid to a vapour when heated. Therefore, camphor can easily be purified by sublimation technique. Sublimation is a phase change in which a solid phase passes directly into the vapor without going through an intermediate liquid phase. Solids which have vapour pressure below melting points can be purified by 1) heating the solid to sublime it 2) condensing the vapor on a cold surface 3) scraping off the condensed solid. Sublimation is not a very accurate method as recrystalization or chromatography. The advantages of sublimation technique is that solvent is not required and not too much transferring is involved, so losses in transfer can be kept low, (John W. Lehman, pg 653). Sublimation is the only new technique used in this experiment, other simple techniques like cooling, temperature monitoring, mixing, addition, weighing, vacuum filtration, drying solids, melting point measurement are also used in this experiment. Results: Mechanism: Calculations: Discussion: The first step in the lab is to set up an apparatus for addition under reflux. This apparatus is comprised of a separatory-addition funnel, claisen adapter & West condenser. Once the apparatus is safely clamped, 1.96g of isorborneol was measured and mixed with 1ml of acetic glacial acetic acid in a 125-ml Erlenmeyer flask. To reiterate, the acetic acid is added to help facilitate the reaction and convert sodium hypochlorite to hypochlorous acid (HOCl). After the acetic acid is added, 5 ml of bleach (NaOCl) is added to the mixture and then placed underneath the previously erected apparatus. 20ml of NaOCl is then placed in the separatory-addition funnel and slowly added to the mixture. The addition is in small portions and the temperature is monitored so as to not allow the temperature of the reaction mixture to exceed 50 degrees Celsius, this is a preventative measure to ensure that there’s no formation of unwanted products such as camphoric acid. The next step would be to test the reaction for excess oxidant. The oxidizing agent in this experiment is HOCl, using KI paper it’s possible to test the solutions’ acidity. Because the solution is acidic the paper will test positive; the iodine on the paper will oxidize and the paper will turn blue. The opposite of this would be the paper turning white indicating a negative result; this is when the solutions is basic or neutral. If the test is negative then NaOCl must be added to maintain an excess of HOCl. The reaction reaches completion after 30 minutes (a white precipitate is formed) and it is at this point where the amount of NaOCl is in solution is 22.5 ml. The solution is then neutralized by adding 3 ml of sodium bisulfate. This is done to ensure that HOCl will not be found in the product. Before filtration, the solution is cooled to 5°C, once this is accomplished vacuum filtration can begin. At the same time the product is washed with cold water in order to remove excess reactants. Once the product is dry it will appear as a solid white compound and will be inserted into a sublimation apparatus. Camphor is known to sublimate from solid to vapour at high temperature. While going under sublimation, camphor is condensed and collected and appears as a white-flaky solid. Once collected the product is weighed to be 0.101 g and the melting point is recorded as 134°C. The mass of isoborneol/ kg of camphor is found to be 17%. The calculated percent yield is 5.3%, therefore, the reaction never reached completion this may be due to not enough kinetic energy being supplied to the solution, the rate at which reactants collide is increased and failure to do so leaves the reactants stationary. A consequence of this is a very slow reaction. Also, since the reaction is kept at low temperature there is less free energy available in order to reach the transition state. Since the mixture did not reach completion, during sublimation, large amounts of isoborneol is found in the final product. Therefore, there exists a 25.1% difference in melting points, it significantly lower than it should be due to impurities in the final product. However, since some of the product was formed, product consisted of 83% camphor. To conclude, the major step in this experiment is ensuring that the reaction goes to completion, if enough energy is not supplied to the reactants the reaction will not go completely forward. This will leave traces of reactants in your final product (impurities) and consequently lower the yield.