Malabsorption in Children
advertisement

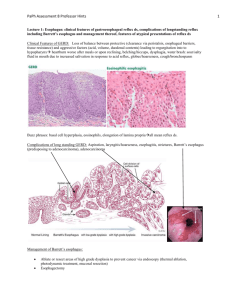
Malabsorption in Children Eyad Altamimi, MD Pediatric Gastroenterologist Assistant Professor of Pediatrics Mu`tah University Malabsorption vs. Maldigestion Decreased intestinal absorption of macronutrients and/or micronutrients “malabsorption” – defect in mucosal phase “maldigestion” – defect in intraluminal phase Normal Digestion and Absorption Mechanical mixing Enzyme and bile salt production Mucosal function Blood supply Intestinal motility Commensal gut flora Fat Digestion and Absorption Carbohydrate and Protein Digestion and Absorption Pancreatic proteases Mucosal peptidases Protein Oligopeptides AA Digestion Absorption Distribution CHO Oligosaccharides Sugars Pancreatic amylase Mucosal disaccharidases Misconception Malabsorption=Chronic Diarrhea But many have loose motions Classification of Malabsorption Luminal Mucosal Postabsorptive Case 1 14 month old F with FTT Watery diarrhea x 2 months; 3-10 episodes per day; stool infectious work-up: negative No nausea, vomiting, abdominal pain, abdominal distention, hematochezia, melena No improvement in symptoms with change to soy milk No significant PMH or FH PE: Pale skin, abdomen distended but soft and nontender, normal bowel sounds, no organomegaly Growth Chart Labs • WBC 14.1, Hgb 9.7, Plt 392; normal MCV and iron studies • AST 30, ALT 23, T bili 0.2, albumin 2.8 • Hemoccult negative, stool WBC negative, spot fecal fat test positive • Celiac Panel – IgA 69 (14-105) – Endomysial AB – Tissue Transglutaminase IgA AB Positive 190.5* (>40) What is Celiac Disease? • Auto-immune condition • Occurs in genetically susceptible individuals • A unique autoimmune disorder because: – Environmental trigger (gluten) and the autoantigen (tissue-transglutaminase) are known – Elimination of the environmental trigger leads to a complete resolution of the disease – Permanent sensitivity to gluten Why is it Important? If untreated it poses long-term adverse health consequences including: Malabsorption Anemia Poor growth Osteopenia Intestinal lymphoma Nutritional Deficiencies Iron, zinc, calcium, Vitamin A, D, E, and K Occurrence Healthy Population: 1:133 First-Degree Relative 1:18 to 1:22 Second-Degree Relative 1:24 to 1:39 Incidence- Females more than males Pathogenesis • Genetic- multi-factorial, polygenic – HLA DQ2 and DQ8 are most common, but, not the only • Over 90% of patients with CD have the DQ2 • Not diagnostic, 40% of population is DQ2+ – We know it is genetic-familial clustering • • • • 10% risk in first degree relatives HLA identical siblings-30% Monozygotic twins 75% Dizygotic twins-10% The Celiac Iceberg Symptomatic Celiac Disease Manifest mucosal lesion Silent Celiac Disease Latent Celiac Disease Genetic susceptibility: - DQ2, DQ8 Positive serology Normal mucosa Clinical Manifestations Gastrointestinal Symptoms (“Classic”) Chronic or recurrent diarrhea Abdominal distention Abdominal pain Vomiting Anorexia Failure to thrive or weight loss Constipation Irritability “Classic” Celiac Disease Non-Gastrointestinal Manifestations Dermatitis Herpetiformis Dental enamel hypoplasia Osteopenia Osteoporosis Short Stature Delayed Puberty Infertility Elevated transaminases Arthritis Neurological Epilepsy Ataxia Neuropathy Dementia Iron Deficiency Anemia Dermatitis Herpetiformis Erythematous macule > urticarial papule > tense vesicles Severe pruritus Symmetric distribution 90% no GI symptoms 75% villous atrophy Abnormal Laboratory Findings in CD Asymptomatic Treatment with a gluten-free diet is recommended for asymptomatic children with proven intestinal changes Asymptomatic patients at risk of osteopenia/osteoporosis Screening in asymptomatic patients: • • • • • • Type 1 diabetes Selective IgA deficiency Down syndrome Turner syndrome Williams syndrome A first degree relative with CD 10-20% 5% 5% 5% 5% 10% Serological Tests Role of serological tests: Identify symptomatic individuals who need a biopsy Screening of asymptomatic “at risk” individuals Supportive evidence for the diagnosis Monitoring dietary compliance Serum IgA Level IgA deficient individuals may have false negative EMA-IgA & TTG-IgA Screen by checking total IgA level or including an IgG based assay EMA-IgG and TTG-IgG) Approximately 6 % of Celiac Disease patients have IgA deficiency Antigliadin Antibodies Antibodies (IgG and IgA) to the gluten protein in wheat, rye and barley Advantages Relatively cheap & easy to perform Improved sensitivity & specificity in young children Disadvantages Poor sensitivity and specificity overall Deamidated Gliadin Antibody • Most recent antibody test available • Antibody response to deamidated gliadin in patients with Celiac Disease seems to be more intense than the antibody response to native gluten (AGA). • Recent studies have shown that anti-TTG still performs better, and it is currently significantly less costly than antiDGP testing. Tissue Transglutaminase – TTG and Endomysial Antibody - EMA • Typically the screening test of choice • Advantages – High sensitivity and specificity (human TTG) • Disadvantages – False negative in young children – Operator dependent – Cost HLA Tests Potential role for DQ2/DQ8 Asymptomatic relatives Trisomy 21, Turner & Williams syndrome Type 1 diabetes Diagnostic dilemmas • TTG +, EMA -, Bx -, Symptoms + Role of Endoscopy • To obtain a small intestinal biopsy for histologic analysis to establish the diagnosis of CD Normal Appearing Scalloping Nodularity Intestinal Histopathology Histologic abnormalities associated with CD are characteristic, but not completely specific. Classically, small intestinal mucosal surface is flattened with absence or marked blunting of intestinal villi Total absorptive surface area is greatly reduced There are typically an increased number of inflammatory cells present in the lamina propria (plasma cells, CD4 cells) Treatment Only treatment for Celiac Disease is a gluten-free diet (GFD) Strict, lifelong diet Avoid: Wheat Rye Barley Oats? Why is Adherence Important? • when children with symptomatic celiac disease adhere to a GFD it results in resolution of GI symptoms, improved growth in height and weight, and normalization of hematological and biochemical parameters • Celiac disease is associated with an overall increased risk of mortality in adults which is primarily the result of GI malignancies. When CD is diagnosed in childhood and GFD is initiated, there appears to be no increased cancer risk and reduced risk of other autoimmune diseases Summary Celiac Disease is a common, subtle enteropathy with variable presentation. Active, appropriate screening is needed to avoid long-term complications of untreated CD. Life long adherence to the diet is important 32 Case 2 An 8 month old child presents with a history of poor growth and a chronic cough He was initially breast-fed, but due to frequent vomiting and loose bowel movements, he was changed to formula feeding, despite trials of different types of formulas (soy, hypoallergenic, etc.), his clinical course was remarkable for bloating, diarrhea and failure to thrive. He developed a daily cough and some respiratory difficulty. At the age of 5 months he was hospitalized for respiratory distress and was diagnosed as having asthma He continued to have loose, large, greasy, foul-smelling stools and failure to thrive He is small for his age HEENT exam is significant for bilateral otitis media and mild nasal congestion Lungs with good aeration and mild wheezing and crepitation Abdomen soft, non-tender, active bowel sounds Color and perfusion are good Sweat test: Weight 120 micrograms; 105 mmol/L (normal <60) Deep throat culture after coughing induced by respiratory therapy using a suction trap collection unit (specimen treated by laboratory as a sputum culture): Klebsiella pneumoniae AST 44 H (normal 0-37), ALT 49 H, (normal l0-40), Alk Phos 324 (normal 104-345) Cystic fibrosis mutation analysis (genetic testing): Positive for one copy of Delta F508 and one copy of R1066C Overview • Cystic fibrosis = abnormal chloride transport caused by mutations in the CF Transmembrane Conductance Regulator Gene (CFTR) • Results in abnormally thick mucous, and secretions in lumens of the body – – – – Lungs Gut Pancreas Biliary tract • Class I-III mutations (ie. delta F508 genotype) typically more severe than Class IV-V mutations Major GI Manifestations Intestinal: GERD; Meconium Ileus; Distal Intestinal Obstruction syndrome (DIOS) Constipation, Intussusception Pancreatic: Pancreatic Insufficiency; Failure to Thrive; Malabsorption of A, D, E, and K vitamins Chronic Pancreatitis, CF Related Diabetes Hepatobiliary: Cirrhosis, Cholelithiasis Steatosis, Portal Hypertension Pancreas: Insufficiency Pancreatic Exocrine Insufficiency • Most common GI complication of CF (85%) • Inadequate pancreatic enzymes = poor nutrient absorption – Fat-soluble vitamin deficiency, steatorrhea, malnutrition • Pancreatic duct obstruction causes acinar cells to replace with adipose fibrotic tissue • Decreased amylase, lipase, colipase, phospholipase – Increased production of salivary amylase, brush border amylase and peptidase, and lingual lipase – Allows adequate monosaccharide and amino acid absorption, and some lipolysis • Decreased bicarbonate – Worsens enzyme deficiency; enzymes optimal in alkalotic environment Pancreas: Insufficiency • Symptoms: foul smelling, bulky, frequent stools – Clinical symptoms with >90% exocrine function lost • Diagnosis: fecal Elastase or 72hr Fecal Fat testing + clinical observations – Fecal Elastase: high sensitivity and specificity for severe PI; performs less well for mild-moderate PI[6] – 72hr Fecal Fat more onerous to perform • Others: fecal chymotrypsin, fecal lipase, secretin stimulated duodenal aspirate Pancreas: Insufficiency • Management – Pancreatic Enzyme Replacement Therapy (PERT) • Pancreatic extract with lipase:protease:amylase • Optimally dissolves in alkaline duodenal fluid – Dose: IU/kg or IU/ mL formula or breast-feed – Dosing is guided by symptoms: diarrhea, bloating, gassiness, foul smelling, greasy stools – Assess for other causes of malabsorption if no response to enzyme therapy Pancreas: Insufficiency • Complications – Prolonged enzyme contact with mucosa may cause ulcers – Excessive doses (>10,000U/kg/d) can cause fibrosing colonopathy (inflammation, strictures)[7] – Inadequate replacement may cause: • Growth failure • Fat soluble vitamin deficiencies (ADEK) • Bone disease (calcium, magnesium, Vitamin D, K) Pancreas: Pancreatitis Pancreatitis • 10% of CF patients rare in insufficiency • Defective pancreatic secretion causes recurrent pancreatitis decline in exocrine function – 20% of patients with pancreatic sufficiency at 1st episode of pancreatitis will go on to pancreatic insufficiency • Rarely 1st presentation of CF Pancreas: CFRD CF Related Diabetes (CFRD) • Exocrine pancreatic insufficiency can evolve to endocrine pancreatic dysfunction • Risk factors: age, pancreatic insufficiency, delta F508 homozygous genotype • 25% by 20 years of age • Insidious onset: poor weight gain/growth, delayed puberty, decline in pulmonary function Summary • CF affects lumens throughout the body • Pancreas: – PI in 85%, high index of suspicion even if clinically asymptomatic – Pancreatitis 10%, rarely in PI – Endocrine pancreatic dysfunction common with age