Neurophysiologie clinique 34 (2004) 33–39
www.elsevier.com/locate/neucli
ORIGINAL ARTICLE
Fear-conditioned cues of impending pain facilitate
attentional engagement
Stefaan Van Damme a,b,*, Jürgen Lorenz c, Christopher Eccleston d,
Ernst H.W. Koster a, Armand De Clercq e, Geert Crombez a,b
a
Department of Psychology, Ghent University, Henri Dunantlaan 2, B-9000 Ghent, Belgium
Research Institute for Psychology and Health, P.O. Box 80.140, 3508 TC Utrecht, The Netherlands
c
University of Hamburg, Institute of Neurophysiology and Pathophysiology, Martinistrabe 52,
20246 Germany
d
Department of Psychology, University of Bath, Claverton Down, Bath BA2 7AY, UK
e
Department of Applied Mathematics and Computer Science, Ghent University, Krijgslaan 281 (S9),
B-9000 Ghent, Belgium
b
Received 7 August 2003; revised and accepted 17 November 2003
KEYWORDS
Selective attention;
Experimental pain;
Classical conditioning
MOTS CLÉS
Attention sélective ;
Douleur
expérimental ;
Conditionnement
classique
Abstract
Aims of study. – Selective attention to signals of impending pain allows the avoidance of
bodily harm. In order to identify the attentional components involved in the selection of
pain signals over competing demands, we used an emotional modification of an exogenous
cueing task.
Methods. – Fifty-two pain-free volunteers detected visual targets of which the location
was correctly or incorrectly predicted by a spatial cue. Cues were emotionally modulated
using differential classical conditioning. The conditioned cue (CS+) was sometimes
followed by an electrocutaneous stimulus (UCS), thus becoming a pain signal, whereas the
UCS never followed the other cue (CS–), referred to as safety signal.
Results. – Analyses of response times showed that pain signals facilitated the directing of
attention to their location in comparison to safety signals. In contrast, pain signals did not
impair disengagement of attention from their location in comparison to safety signals.
Conclusion. – It is concluded that attention is more strongly engaged to a signal of
impending pain compared with a cue signalling its absence. We explore why disengagement from the pain signal is not impaired compared to the safety signal. The findings are
discussed in terms of the defensive importance of pain anticipation.
© 2003 Elsevier SAS. All rights reserved.
Résumé
Objectifs. – L’attention sélective dirigée vers un signal de la douleur imminente permet
d’éviter les dommages physiques. Afin d’identifier les composantes attentionnelles
impliquées dans la sélection des signaux de la douleur, nous avons utilisé une version
émotionnelle de la tâche d’indiçage.
Méthodes. – Cinquante-deux sujets volontaires sains avaient pour tâche de détecter des
cibles visuelles, dont la localisation spatiale était annoncée — de façon correcte ou
incorrecte — par un indice spatial. La valence émotionnelle de l’indice était induite à
* Corresponding author. Faculty of Psychology and Educational Sciences, Ghent University, Henri Dunantlaan 2, 9000 Ghent,
Belgium. Tel.: +32-9-2649105; fax: +32-9-2649149.
E-mail address: Stefaan.VanDamme@UGent.be (S. Van Damme).
© 2003 Elsevier SAS. All rights reserved.
doi: 10.1016/S0987-7053(03)00102-3
34
S. Van Damme et al.
l’aide d’une procédure de conditionnement classique. Lors de certains essais, l’indice
conditionné (CS+) était suivi d’un stimulus électrique nociceptif (UCS), devenant alors un
signal de douleur. En revanche, aucun UCS ne suivait l’indice non conditionné (CS–), qui
devenait alors un signal de sécurité.
Résultats. – L’analyse des temps de réaction a montré que le signal de douleur facilitait
la direction de l’attention vers sa localisation spatiale, en comparaison au signal de
sécurité. En revanche, le signal de douleur ne détériorait pas le désengagement de sa
localisation spatiale, par rapport au signal de sécurité.
Conclusion. – Nous concluons que l’attention est attirée plus fortement par un signal de
douleur imminente en comparaison à un signal annonçant l’absence de douleur. Nous
explorons pourquoi le désengagement du signal de douleur n’est pas détérioré en
comparaison au signal de sécurité. Les résultats sont discutés en termes de caractère
défensif de l’anticipation de la douleur.
© 2003 Elsevier SAS. All rights reserved.
Introduction
The question of how pain attracts attention over
competing demands has become a substantial research topic over the last decade. There is already
a significant amount of research documenting the
effects of pain on attention. The idea that pain
demands attention, interrupts ongoing activities,
and interferes with present demands has been supported by numerous studies in both clinical and
non-clinical populations. These studies feature a
variety of research methods, such as behavioural
tasks [2,5,12,29,33], psychophysiological measures
[9,10,19,20], and functional brain imaging [1,8,24].
Eccleston and Crombez [13] developed a
cognitive-affective model in which they argued
that the interruption of attention by pain is a
normal and evolutionary adaptive process. According to these authors, pain has a processing priority
by activating a primitive defensive system that
urges escape from somatic threat. Although pain
can occur without warning, the learning of signals
of impending pain significantly contributes to this
protective function. For example, when we hear
the humming of a wasp while eating a fruit, our
attention will be immediately drawn to the threat
of a painful sting, and we will attempt to escape
from this situation. The idea that pain anticipation
subserves an important protective function has
been supported by neurophysiological research in
both clinical and non-clinical populations. For instance, in a number of functional brain imaging
studies, changes in activity of cortical nociceptive
networks were measured during anticipation of
pain and during actual painful stimulation. It was
found that cortical networks involved in the processing of pain itself were already activated during
anticipation of pain, suggesting that these locations
are directly affected by cognitive factors such as
attention [25,26]. However, little is known about
the mechanisms by which pain signals capture attention, facilitating the detection of pain itself.
Dawson et al. [6] investigated the allocation of
attention to signals of impending pain. They asked
participants to concentrate on the differential conditioning of visual stimuli. The conditioned stimulus
(CS+) was frequently followed by an electrical
shock, thus becoming a pain signal. The other
stimulus (CS–) was never followed by an electrical
shock. Furthermore, participants were asked to
perform an auditory reaction time task as a secondary task. The authors found that reaction times to
auditory stimuli were significantly slower during
CS+ presentation than during CS– presentation, suggesting an increased allocation of cognitive resources to pain signals [6]. Because this study measured general attentional deployment through the
interference of task performance by pain signals, a
more detailed understanding of the attentional
sub-components involved was not possible.
In general, three components of attention to all
forms of threat can be distinguished [14,15,23,33]:
(1) an initial transient shift of attention to a threatening stimulus, (2) a sustained engagement with
the threatening stimulus, and (3) disengagement
from the threatening stimulus. In order to differentiate these attentional components during the processing of pain signals, we used an emotional modification of an exogenous cueing paradigm,
originally developed by Posner et al. [27]. In an
exogenous cueing paradigm, participants are asked
to detect a visual target presented at the left or
right side of a fixation cross. Targets are preceded
by a visual cue at the same spatial location (validly
cued trials) or at the opposite location (invalidly
cued trials). Presenting a spatial cue facilitates or
inhibits target detection in comparison with a condition in which no spatial cues are presented: the
presentation of a valid cue typically leads to response time benefits (due to attentional engage-
Fear-conditioned cues of impending pain facilitate attentional engagement
ment at the correctly cued location), whereas the
presentation of an invalid cue leads to response
time costs (due to attentional disengagement from
the incorrectly cued location), described as the cue
validity effect [27]. In the present study, spatial
cues are emotionally modulated by a differential
classical conditioning procedure. A nociceptive
stimulus (UCS) sometimes follows the conditioned
cue (CS+), rendering it a pain signal. In contrast,
the UCS never follows the other cue (CS–), which we
refer to as the “safety signal” according to Seligman’s hypothesis [28]. Using this paradigm we hypothesize that two components of attention to
signals of impending pain will be differentiated:
First, we expect facilitated attentional engagement to pain signals, indicated by stronger response time benefits when the CS+ in comparison
with the CS– is used as a valid spatial cue. Second,
difficulty disengaging from pain signals should be
indicated by stronger response time costs when the
CS+ in comparison with the CS– is used as an invalid
spatial cue.
Until now, most studies investigated the effect
of pain on attention. The present study is one of the
first to investigate the effect of pain anticipation
on spatial attention. Furthermore, the present
study differs from previous work in providing a
detailed examination of the underlying attentional
components.
Method
Participants
Fifty-two undergraduate psychology students (eight
males and 44 females; mean age = 19.04 years)
participated to fulfil course requirements. All participants gave their informed consent and were free
to terminate the experiment at any time. Each
person had normal or corrected-to-normal eyesight. Experimental duration was approximately
30 min.
Apparatus and test material
The exogenous cueing task was programmed and
presented by the INQUISIT Millisecond software
package (Inquisit 1.32, 2001) on a S710 Compaq
Deskpro computer with a 72 Hz, 17-inch color monitor. INQUISIT measures response times with a millisecond accuracy [7].
Target stimuli consisted of black squares (1.1 by
1.1 cm), presented on a white background. Two
colour slides (green and pink; 4.8 cm high × 6.5 cm
wide) were used as spatial cues for the location of
35
the targets. Each trial began with a fixation cross in
the middle of the screen (duration of 1000 ms).
Cues were presented 9.2° from the fixation cross
for a duration of 200 ms. Target onset followed
immediately after cue offset. On half of the test
trials, cue location correctly predicted target location (validly cued trials). On the other half of the
test trials, cue location incorrectly predicted target
location (invalidly cued trials). Participants were
seated 60 cm from the computer screen. They were
instructed to respond to the left targets by pressing
the ‘q’ key with the left index finger and to the
right targets by pressing the ‘5’ key with the right
index finger on a standard AZERTY computer keyboard. A trial ended when a participant responded
or 2000 ms had elapsed. In order to control for
responses to cues instead of targets, a number of
trials were presented, in which the cue was not
followed by a target (catch trials). Furthermore, in
order to ensure that participants maintained gaze
at the middle of the screen, a number of control
trials were presented. In these trials, the fixation
cross was followed only by a randomly selected
digit between 1 and 9 for a duration of 50 ms (digit
trials). Participants were instructed to report the
digit aloud. If participants were not able to report
the digits (correctly), this indicated that they did
not maintain gaze at the fixation cross.
Cues were emotionally modulated by a differential classical conditioning procedure. The conditioned cue (CS+) was on one third of the presentations followed by a nociceptive stimulus (UCS). The
other cue (CS–) was never followed by an UCS. The
UCS was a transcutaneous electrocutaneous stimulus, delivered by a Digitimer constant current
stimulator (Digitimer DS7A, 1998). Intensity of the
electrocutaneous stimulus was selected individually by each participant (see below). Electrocutaneous stimuli had an instantaneous rise and fall
time, and a duration of 750 ms. The stimuli were
delivered by two lubrificated Fukuda standard
Ag/AgCl electrodes (1 cm diameter) attached to
the external side of the right ankle. The skin at the
electrode sites was first abraded with a peeling
cream (Nihon Kohden) to reduce skin resistance.
Which colour was CS+ or CS– was counterbalanced
across participants. The CS+ and CS– were presented equally often, in a fixed random order with
no more than three consecutive presentations of
one cue.
Procedure
Participants were tested individually in a soundattenuated room designed for psychophysiological
experiments.
36
S. Van Damme et al.
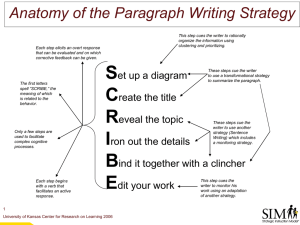
Figure 1 Schematic illustration of the four trial types. On each trial, a fixation cross was presented for 1000 ms. This was immediately
followed by the presentation of a cue (200 ms duration). Immediately after cue offset the target was introduced. Targets were
presented at the same location of cues (validly cued trials), or at the opposite location of cues (invalidly cued trials). The CS+ was
followed by the UCS in one third of the trials.
Preparation phase
Participants were informed that an electrocutaneous stimulus would be used during the experiment.
They were further informed that this stimulus
“stimulates the pain fibres and that most people
find this kind of stimulation unpleasant”. After this,
participants gave informed consent. To familiarise
the participants with the electrocutaneous stimuli,
they were given a series of stimuli with increasing
intensity. They were asked to select a stimulus
which they found aversive but tolerable, and which
required a certain effort to deal with. The electrocutaneous stimulus chosen by the participant was
set as the intensity used during the experiment.
Practice phase
Participants were seated in front of a computer to
perform the exogenous cueing task. All instructions
were presented on the computer screen. Participants were instructed to respond to the targets as
quickly as possible, without sacrificing accuracy.
Participants were informed about cues and targets,
and they were asked to respond only to the location
of the targets by pressing on the corresponding key.
Participants were told that cues would predict target location on some of the trials. Furthermore,
they were informed that there would be trials on
which only a cue but no target would appear and
they were asked not to respond on these trials. They
were told that there would also be trials in which
only a digit would appear which they had to report
aloud. The practice phase consisted of 10 trials:
four validly cued trials, four invalidly cued trials,
one catch trial, and one digit trial. Participants
were made aware of the fact that no electrocutaneous stimuli would be presented during this phase.
Experiment phase
Participants were informed that one type (colour)
of cue would sometimes be followed by an electrocutaneous stimulus, and that the other type of cue
would never be followed by an electrocutaneous
stimulus. Participants did not know in advance
which cue would be followed by the electrocutaneous stimulus. The experiment phase consisted of
165 trials: 72 validly cued trials, 72 invalidly cued
trials, 12 catch trials, and nine digit trials. One
third of the CS+ presentations was followed by an
electrocutaneous stimulus (UCS). These trials were
not analysed in order to make sure that the effect
of the CS+ was not confounded by the effect of the
UCS. The experiment phase started with two buffer
trials in which the CS+ was followed by an UCS in
order to facilitate the differential conditioning.
Fig. 1 shows a schematic illustration of the four trial
types used in the study.
Post-experiment phase
As a method check, participants rated to what
extent they expected an electrocutaneous stimulus
following the CS+ and the CS– on an 11-point nu-
Fear-conditioned cues of impending pain facilitate attentional engagement
merical graphic rating scale (anchored zero = not at
all to 10 = very strongly). On a similar scale, participants also rated how aversive they found the electrocutaneous stimulus.
Statistical analyses
A 2 (cue validity: valid, invalid) × 2 (signal: CS+,
CS–) ANOVA with repeated measures was performed
upon the mean response times (RT). Responses
faster than 150 ms were considered as responses to
cues instead of targets, and were removed from the
RT analyses. Greenhouse-Geisser corrections (with
corrected degrees of freedom) are presented
where the sphericity assumption is violated
(Mauchly’s test of sphericity; P < 0.05).
Results
Participants rated the electrocutaneous stimulus as
aversive (M = 6.38, S.D. = 1.51). The differential
conditioning procedure was successful. Participants expected the electrocutaneous stimulus significantly more after the presentation of a CS+
(M = 6.94, S.D. = 2.22) than after the presentation
of a CS– (M = 0.71, S.D. = 1.64), t(51) = 14.72,
P < 0.001.
The mean percentage of error rates was rather
small for responses to validly cued CS+ trials
(0.62%), validly cued CS– trials (0.44%), invalidly
cued CS+ trials (2.96%), and invalidly cued CS– trials
(1.18%). Participants responded (erroneously) to
3.37% of the catch trials. They complied with gaze
fixation, consistently reporting the digits. All error
trials (1.31%) and outliers (RTs less than 150 ms or
greater than 750 ms; 1.03%) were removed from the
RT analyses. Also the trials in which the CS+ was
followed by an electrocutaneous stimulus (UCS)
were removed from the analyses, in order to prevent that the effect of the UCS would confound the
effect of the CS+. The mean RT was then calculated
for each participant as a function of each factor in
the design.
A 2 (cue validity: valid, invalid) × 2 (signal: CS+,
CS–) ANOVA with repeated measures showed a significant main effect of cue validity, F(1.51) = 30.59,
MSE = 548.24, P < 0.001, indicating that responses
to validly cued targets were faster than responses
to invalidly cued trials (331 vs. 349 ms). The main
effect of signal was not significant, F(1.51) = 2.34,
MSE = 238.13 ns. Of particular importance for this
study was the significant interaction effect of cue
validity × signal, F(1.51) = 5.93, MSE = 214.93,
P < 0.05. This interaction was further analysed in
terms of engagement and disengagement effects.
37
The engagement component relates to response
times on validly cued trials. In particular, we tested
whether the presentation of the CS+ as valid cue
facilitated target detection compared to the presentation of the CS– as a valid cue. The disengagement component covers response times on invalidly
cued trials. We tested whether the presentation of
the CS+ as an invalid cue inhibited target detection
compared to the presentation of the CS– as an
invalid cue. All tests were done using t-tests for
dependent samples.
We found that the presentation of pain signals as
spatial cues affected the engagement component
but not the disengagement component. Responses
were significantly faster on trials with the CS+ as a
valid cue (M = 327 ms, S.D. = 41 ms) compared to
trials with the CS– as a valid cue (M = 336 ms,
SD = 46 ms), t(51) = 2.52, P < 0.05. However,
response times did not differ on trials with the CS+
as an invalid cue (M = 350 ms, S.D. = 47 ms)
compared to trials with the CS– as an invalid cue
(M = 349 ms, S.D. = 49 ms), t(51) = 0.65 ns.
Discussion
The main objective of this study was to investigate
the mechanisms by which pain signals capture attention. We characterized pain signals as stimuli
that signal the occurrence of pain, whereas we
considered stimuli signalling the absence of pain as
safety signals. We used both of these stimuli in an
emotional modification of the exogenous cueing
paradigm and hypothesized that two attentional
components could be identified: (1) Pain signals
may facilitate attentional engagement by accelerating covert orienting to the target location when
compared with safety signals (engagement component). (2) Pain signals may inhibit attentional disengagement by slowing covert orienting to targets
in the opposite location when compared with safety
signals (disengagement component).
First, we replicated the cue validity effect: Presenting a valid spatial cue produced covert orienting of attention to the cue location and accelerated
target detection [27]. Notably, we obtained a robust cue validity effect although we used an equal
number of correctly and incorrectly cued trials. In
original experiments, valid and invalid cues had
occurrence probabilities of 80% and 20% of the
trials, respectively [27]. Second, and of most importance to this study, we observed a differential
modulation of attentional engagement and disengagement by the signal value of the cues: the pain
signal induced stronger attentional engagement
relative to the safety signal, whereas both pain and
38
safety signals were equally difficult to disengage
from.
The differential effect of pain signals on engagement and disengagement is intriguing and needs
further consideration. First, it appears reasonable
that the attentional engagement component in the
exogenous cueing task benefits from threatening
cues due to their greater perceptual salience and
induction of more arousal than neutral cues. However, a limitation of the present study was that no
physiological measures were included, which could
have clarified the role of arousal. Second, strong
engagement might generally be facilitated by
simple warning signals that allow rapid processing.
Consistent with this view, LeDoux [18] argued that
the simplicity of conditioned stimuli contributes to
the efficiency of recruitment of the brain network
underlying fear-conditioning. Thus, it appears that
the phasic engagement process benefits from the
simple and alerting character of the pain signal.
The biological value of this function may be seen in
the heightened spatial awareness of a potential
threat that needs to be rapidly defended. In contrast, disengagement involves a release from an
ongoing tonic process. The pain signal, due to its
short-lived duration and due to the brevity and low
cognitive demand of the electrocutaneous stimulus
that it signals, may have failed to sufficiently intensify this tonic process to render disengagement
impaired. This would explain why attentional disengagement is affected by more complex cognitive
cues such as pictures or words, as has been demonstrated in a number of recent studies using a crossmodal cueing paradigm [33,34].
Further support for a dissociation of attentional
engagement and disengagement is provided by neuroimaging studies. A cortical network consisting of
frontal, parietal, and cingulate regions has been
described to play a key role in identifying and
shifting attention to salient features of the sensory
environment [11]. By rendering the cue a pain
signal, this network might have been more strongly
recruited, facilitating target detection within the
cued location. In contrast, holding of attention over
prolonged periods of time has been suggested to
rely on sustained activity in the basal ganglia and
prefrontal cortex [3,4]. These regions have been
described to be active during sustained painful
stimulation in humans [21]. More particularly, the
left dorsolateral prefrontal cortex has been suggested to be specifically involved in the disengagement from affective pain processing [22].
Our findings are partly in contrast with previous
studies using an emotional modification of the exogenous cueing paradigm. One reason for discrepancy may be that most studies used threatening
S. Van Damme et al.
words [15,32] or pictures [15,16,35] as cues, which
involve more complex cognitive and emotional processing, influencing disengagement (see above).
Another reason may be that none of these studies
used paradigms with pain stimuli or conditioning.
More similar to our study are exogenous cueing
paradigms that have used a fear-conditioning procedure with aversive noise [30,31]. However, in
these studies, the exogenous cueing task was not
performed during acquisition as in our study, but
only during extinction. The apparent discontinuity
between acquisition and extinction might have accounted for the contrasting results in these studies
[17].
It can be concluded that attention is more
strongly engaged to a signal of impending than to a
cue signalling the absence of pain. From an evolutionary perspective, one can argue that this process
may subserve the function of locating the potential
risk and adopting protective behaviours before an
injury occurs. However, it may become maladaptive in situations of chronic pain where there is no
behavioural escape from pain. In the general context of attention to the threat of pain and the
initiation of avoidance behaviour, it is therefore
necessary to disentangle the diverse components of
attentional shift, engagement and disengagement
[14,15,23,33]. Our study is one of the first that
allows such detailed investigation of the underlying
processes involved in spatial attention to pain signals. One limitation of the present study is that we
did not use control trials with non-nociceptive
stimuli. Further studies may use a non-painful somatosensory stimulus of similar salience [34] in
order to investigate whether our effects are unique
to pain. In future research, it may also be useful to
create experimental paradigms that allow a direct
investigation of the link between spatial attention
to pain signals and avoidance behaviour. Finally,
our modulation of an attentional cueing task by a
fear-conditioning procedure may be combined in
future experiments with neurophysiological measures (EEG or fMRI) to foster our understanding of
the differential anatomical representation of attentional engagement to and disengagement from
pain.
Acknowledgements
This study was supported by a research grant
(G.0107.00) of the Fund for Scientific Research,
Flanders (Belgium) to Geert Crombez. The authors
wish to thank Jan De Houwer and Bruno Verschuere
for their helpful comments on earlier drafts of the
manuscript, and Valéry Legrain for helping with the
French summary.
Fear-conditioned cues of impending pain facilitate attentional engagement
References
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
[12]
[13]
[14]
[15]
[16]
[17]
Aziz Q, Schnitzler A, Enck P. Functional neuroimaging of
visceral sensation. J Clin Neurophysiol 2000;17:604–12.
Bushnell MC, Duncan GH, Dubner R, Jones RL, Maixner W.
Attentional influences on noxious and innocuous cutaneous
heat detection in humans and monkeys. J Neurosci 1985;
5:1103–10.
Corbetta M, Miezin FM, Dobmeyer S, Shulman GL,
Petersen SE. Selective and divided attention during visual
discriminations of shape, color, and speed. J Neurosci
1991;11:2383–402.
Coull JT, Frith CD, Buchel C, Nobre AC. Orienting attention
in time: behavioural and neuroanatomical distinction
between exogenous and endogenous shifts. Neuropsychologia 2000;36:808–19.
Crombez G, Baeyens F, Eelen P. Sensory and temporal
information about impending pain: the influence of predictability on pain. Behav Res Ther 1994;32:611–22.
Dawson ME, Schell AM, Beers JR, Kelly A. Allocation of
cognitive processing capacity during human autonomic
classical conditioning. J Exp Psychol 1982;111:273–94.
De Clercq A, Crombez G, Roeyers H, Buysse A. A simple and
sensitive method to measure timing accuracy. Behav Res
Meth Ins C 2003;35:109–15.
Derbyshire SWG, Vogt BA, Jones AKP. Pain and stroop
interference tasks activate separate processing modules in
anterior cingulate cortex. Exp Brain Res 1998;118:52–60.
Dowman R. Attentional set effects on spinal and supraspinal responses to pain. Psychophysiol 2001;38:451–64.
Dowman R, Shell S. The pain-related negative difference
potential: a direct measure of central pathway activity or
of interactions between the innocuous somatosensory and
pain pathways. Neurophysiol Clin 1999;29:423–42.
Downar J, Crawley AP, Mikulis DJ, Davis KD. The effect of
task relevance on the cortical response to changes in visual
and auditory stimuli: an event-related fMRI study. Neuroimage 2001;14:1256–67.
Eccleston C. Chronic pain and attention: a cognitive
approach. Br J Clin Psychol 1994;33:535–47.
Eccleston C, Crombez G. Pain demands attention: a
cognitive-affective model on the interruptive function of
pain. Psychol Bull 1999;125:356–66.
Eysenck MW. Anxiety: the cognitive perspective. Hillsdale:
Lawrence Erlbaum Associates; 1992 195 p.
Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli
draw or hold attention in subclinical anxiety? J Exp Psychol
2001;130:681–700.
Fox E, Russo R, Dutton K. Attentional bias for threat:
evidence for delayed disengagement from emotional
faces. Cogn Emot 2002;16:355–79.
Hugdahl K. Cortical control of human classical
conditioning: automatic and positron emission tomography
data. Psychophysiology 1998;35:170–8.
39
[18] LeDoux J. Emotion: clues from the brain. Annu Rev Psychol
1995;46:209–35.
[19] Legrain V, Guérit J, Bruyer R, Plaghki L. Attentional modulation of the nociceptive processing into the human brain:
selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain 2002;99:21–39.
[20] Lorenz J, Bromm B. Event-related potential correlates of
interference between cognitive performance and tonic
experimental pain. Psychophysiology 1997;34:436–45.
[21] Lorenz J, Cross DJ, Minoshima S, Morrow TJ, Paulson PE,
Casey KL. A unique representation of heat allodynia in the
human brain. Neuron 2002;35:393–393.
[22] Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind:
the role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003;126:1079–91.
[23] Mogg K, Bradley BP. A cognitive-motivational analysis of
anxiety. Behav Res Ther 1998;36:809–48.
[24] Peyron R, Laurent B, Garcia-Larrea L. Functional imaging
of brain responses to pain. A review and meta-analysis.
Neurophysiol Clin 2000;30:263–88.
[25] Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, et al. Dissociating pain from its anticipation in
the human brain. Science 1999;284:1979–81.
[26] Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P,
Maieron M, et al. Does anticipation of pain affect cortical
nociceptive systems? J Neurosci 2002;22:3206–14.
[27] Posner MI, Inhoff A, Friedrich FJ, Cohen A. Isolating attentional systems: a cognitive-anatomical analysis. Psychobiology 1987;15:107–21.
[28] Seligman MEP. Chronic fear produced by unpredictable
shock. J Compar Physiol Psychol 1968;66:402–11.
[29] Spence C, Bentley DE, Phillips N, McGlone FP, Jones AKP.
Selective attention to pain: a psychophysical investigation.
Exp Brain Res 2002;145:395–402.
[30] Stormark KM, Hughdahl K. Peripheral cueing of covert
spatial attention before and after emotional conditioning
of the cue. Int J Neurosci 1996;86:225–40.
[31] Stormark KM, Hughdahl K, Posner MI. Emotional modulation of attention orienting: a classical conditioning study.
Scand J Psychol 1999;40:91–9.
[32] Stormark KM, Nordby H, Hughdahl K. Attentional shifts to
emotionally charged cues: behavioural and ERP data. Cogn
Emot 1995;9:507–23.
[33] Van Damme S, Crombez G, Eccleston C. Retarded disengagement from pain cues: the effects of pain catastrophizing and pain expectancy. Pain 2002;100:111–8.
[34] Van Damme S, Crombez G, Eccleston C, Goubert L.
Impaired disengagement from threatening cues of impending pain in a crossmodal cueing paradigm. Eur J Pain [in
press].
[35] Yiend J, Mathews A. Anxiety and attention to threatening
pictures. Q J Exp Psychol A 2001;54:665–81.