Ben Holston
advertisement
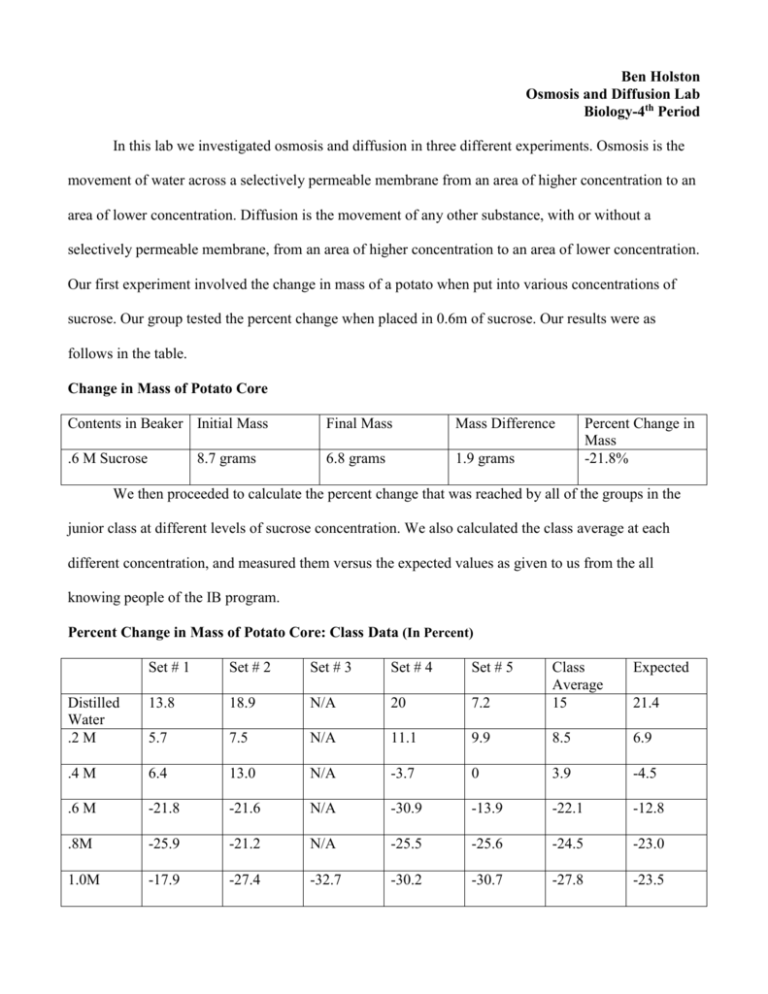
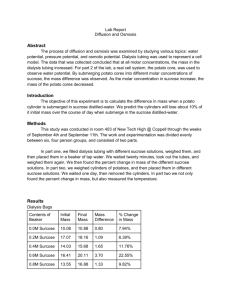
Ben Holston Osmosis and Diffusion Lab Biology-4th Period In this lab we investigated osmosis and diffusion in three different experiments. Osmosis is the movement of water across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. Diffusion is the movement of any other substance, with or without a selectively permeable membrane, from an area of higher concentration to an area of lower concentration. Our first experiment involved the change in mass of a potato when put into various concentrations of sucrose. Our group tested the percent change when placed in 0.6m of sucrose. Our results were as follows in the table. Change in Mass of Potato Core Contents in Beaker Initial Mass Final Mass Mass Difference .6 M Sucrose 6.8 grams 1.9 grams 8.7 grams Percent Change in Mass -21.8% We then proceeded to calculate the percent change that was reached by all of the groups in the junior class at different levels of sucrose concentration. We also calculated the class average at each different concentration, and measured them versus the expected values as given to us from the all knowing people of the IB program. Percent Change in Mass of Potato Core: Class Data (In Percent) Set # 1 Set # 2 Set # 3 Set # 4 Set # 5 Expected 7.2 Class Average 15 Distilled Water .2 M 13.8 18.9 N/A 20 5.7 7.5 N/A 11.1 9.9 8.5 6.9 .4 M 6.4 13.0 N/A -3.7 0 3.9 -4.5 .6 M -21.8 -21.6 N/A -30.9 -13.9 -22.1 -12.8 .8M -25.9 -21.2 N/A -25.5 -25.6 -24.5 -23.0 1.0M -17.9 -27.4 -32.7 -30.2 -30.7 -27.8 -23.5 21.4 The following is a graph of three different lines showing percentage change in potato core. These lines are the 4th period values (groups 1-3), the junior class values and the expected values. Percent Change in Mass of Potate Cores 30 20 Percent Change 10 0 0 0.2 0.4 0.6 0.8 1 4th Period Avg Class Avg Expected -10 -20 -30 -40 Molarity What this information shows us is that the potato had a sucrose molarity of around 0.4 because at that value the potato started to lose mass via osmosis rather than gain it. At molarities of a lower number the potato would take on mass because it would be absorbing water. This was because the concentration of sucrose was so low that there was higher concentration of water outside of the potato then there was in the potato. However, when the concentration of sucrose increased to above 0.4 the concentration of water in the potato was higher, which led to a decrease in the mass of the potato. While we did have four different groups working on the different sucrose concentrations we still had many class averages that were nowhere near the expected average. There are many weaknesses and limitations that could have led to this problem. To start, it was nearly impossible for each potato core to be similar in size. They could be very small to very large, and this could effect the percent change in the potato. Also, there could be different levels of water concentration throughout the potato, a core from the edge of the potato could have a different concentration then a core from the middle, potentially giving us misleading information. We were also rushed through this lab to successfully complete within the time period, this could have forced some of us to make errors. The solutions were arranged in a confusing manner, and if a solution was taken and then replaced in a differently labeled spot it could lead to another group using the wrong solutions. To address these problems I would suggest having all of the potato cores cut by the same person from the same part of the potato, to ensure that there were no variables being affected there. Also, I would try to make it clearer which solution belonged where, so that there would be no errors from using the wrong solution. As always, more time would always be helpful to cut down on human error in the lab. The second experiment we did involved viewing the structure of cells under a microscope. Initially we measured an onion cell in normal conditions under a microscope and drew what we saw (this is turned in with the raw data). After this we added NaCl to the cell and watched what happened. In the original view the cells were intact, but after adding NaCl we saw cell walls begin to break down and blend together. We flushed the NaCl out with water, and after this the cell walls started to rebuild. Again, all drawings are attached. We can conclude that the NaCl is a substance that breaks down cell walls based on this experiment. We also learned that cell walls will replenish after this is removed, an important factor in the survival of cells. There were, however, many problems with this experiment. One problem was that we did not have as many cells as we would have liked, and so it became very difficult to view the cell under the microscope at times. Also, the change in cells was very small and hard to notice. The cells potentially could have remained in tact and just appeared blurred under the NaCl concentration. Our group had to visit other groups microscopes to view cells that were clear, and we have no idea if these groups made errors in their experiment because we weren’t working with them. A thicker slice of onion to view the cells would have helped greatly, and also more magnification so that we could be certain that the cell walls were indeed breaking down, and there wasn’t another factor. The final experiment we did involved a dialysis bag. In a similar experiment to the potato core we filled up a dialysis bag with a certain concentration of glucose. We then placed this bag into a solution of water and iodine and observed what happened. My group was again responsible for the 0.6 M solution, which is what we placed in our dialysis bag, the results are as follows. Change in Mass of Dialysis Bag Contents in Bag Initial Mass Final Mass Mass Difference .6 M Sucrose 22 grams 24 grams 2 grams Percent Change in Mass 9.1% After our individual experiment we again compiled class data of all of the percent changes that occurred in the various groups and at various concentrations. Percent Change in Mass of Dialysis Bag: Class Data in Percent Set # 1 Set # 2 Set # 3 Set # 4 Set # 5 Expected -1.2 Class Average -2.7 Distilled Water .2 M -4.2 0 N/A 0 0 4.0 N/A 4.4 1.5 3.3 3.1 .4 M 7.7 8.0 N/A 8.6 11.1 8.9 7.7 .6 M 9.1 6.8 N/A 11.7 N/A 9.2 11.0 .8M 10.7 11.1 N/A 16 16 13.5 14.8 1.0M 14.8 19.1 8.2 12 20 14.8 18.2 1.2 We can conclude based on this experiment that the higher the concentration of glucose in the bag the more the bag will absorb. This happens because the concentration of iodine outside the bag was always greater, meaning that it would flow into the dialysis tubing and increase the mass. There was also enough concentration of water outside the bag for that to enter as well, which is why it increased in every situation. As the concentration of glucose in the bag went up this meant that there was a higher and higher concentration of other substances outside the bag, leading to more and more gains in the mass of the dialysis tubing. The class average was much closer to the expected numbers in this experiment, but there were still things that could have gone wrong. It was difficult to tie a very tight not in the bottom of the tube, and this led to problems with leaking out of the bag. One group had a black precipitate in their bag, which was caused by a reaction of the iodine and the starch, there result had to be scrapped because of this error. If this had happened more frequently it would have been difficult to compile accurate data from the experiment. Also, there was the potential that we did not allow enough time for diffusion, because we rushed through the phase of the experiment and may not have left a full half hour for it to develop. To fix these problems we should probably find a better way to seal the bag besides tying it, because this can lead to leakage. Also, maybe a substance besides starch that wouldn’t react with iodine but not hurt the experiment would be very helpful. Again, more time could have given us better information about what was happening in the dialysis tubing.