The Formula For A Hydrate
advertisement

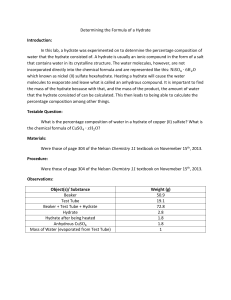
The Formula For A Hydrate I. Objectives: In this lab you are going to calculate the percent by mass of water in copper (II) sulfate and then calculate the empirical formula for the hydrate. II. Materials: Copper (II) sulfate Crucible tongs Hanging pan balance evaporating dish Bunsen Burner dropping pipet Ring Stand Mesh Wire Pre Lab Questions: 1. What is a hydrate? 2. What is an anhydrous compound? 3. What does the name of a hydrate indicate about its composition? 4. If 11.75 grams of a hydrate of Cobalt (II) chloride is heated, 9.25 grams of anhydrous compound remains. What is the formula and name for this hydrate? 5. A 1.628 gram sample of a hydrate of magnesium iodide is heated until its mass is reduced to 1.072 grams and all the water has been removed. What is the formula of the hydrate? III. Procedure: 1. Obtain a pair of safety glass from the back of the room. 2. Copy down the table used to record observations and the table used to record data. 3. Make observation about the appearance of the hydrate before heating. Record in observation table. 4. Measure the mass of a clean dry evaporating dish. Record your data. 5. Add enough copper (II) sulfate hydrate to cover the bottom of the evaporating dish. This should be about one teaspoonful. (best results if you use under 3.0 grams) 6. Measure the mass of the evaporating dish and the hydrate. Record your data. 7. Set the evaporating dish with the hydrate on a wire mesh and begin heating. Heat very slowly and do not over heat the hydrate. To make sure the hydrate is heating evenly, move the Bunsen burner periodically. While the hydrate is heating observe any changes in appearance. Record observations in table 1. 8. Stop heating when you see that there are no other changes (this will take about 10 to 15 minutes. If you start to see black around the edge of hydrate STOP, it is starting to burn). You now have the anhydrous form of copper (II) sulfate in the evaporating dish. 9. Using a pair of tongs, set the evaporating dish on the table top. Allow to cool for approximately 5 minutes, then place on balance using your tongs. Record the mass of the evaporating dish and anhydrous copper (II) sulfate. Record in table 2. 10. Fill a pipet with water and add several drops to the anhydrous chemical. Record your observations as the hydrate reforms. Notice the temperature change by feeling the evaporating dish. Record your observations. 11. Wipe Copper Sulfate into the trash can. Then wash and return evaporating dish to shelf. IV. Data and Analysis: Observation table 1 Hydrate before heating Heating the hydrate Adding several drops of water to the anhydrous chemical. Data Table 2 Mass of evaporating dish Mass of evaporating dish with hydrate Mass of hydrated CuSO4 Mass of evaporating dish and anhydrous compound Mass of anhydrous compound Mass of water removed from original CuSO4 hydrate V. Calculations: 1. Use the experimental data to calculate the formula for hydrated copper (II) sulfate. Show All Work! VI. Conclusion: 1. Describe what effect loss of product (described above) might have on final results. Be specific in describing whether you think this would make your final weight too high or too low, and how this might affect your conclusions. 2. Why is it necessary to let the crucible cool before measuring the mass? 3. Why does copper (II) sulfate change colors when heated? 4. Copper (II) sulfate in its anhydrous form will slowly absorb moisture from the atmosphere. It turns into copper (II) sulfate hydrate. What change in appearance will be observed as this happens?