SBI4U - MsKhan
advertisement

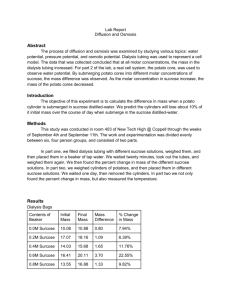
SBI4U Name: _______________ Factors Affecting the Rate of Osmosis In this investigation, you will determine how environmental factors such as solute concentration and temperature affect the rate of osmosis through dialysis tubing. The investigation is divided into two parts: Part 1 and Part 2. In Part 1, you will investigate the effect changes in solute concentration have on the rate of osmosis. In Part 2, you will investigate the effect of changes in temperature. A step-by-step procedure is provided for Part 1. You will design and carry out the procedure for Part 2. Materials Needed: safety goggles distilled water 40% sucrose stock solution 5 250-ml beakers 10mL graduated cylinder electronic scale 5 pieces of dialysis tubing, each about 15 cm long paper towel transfer pipettes masking tape for labels funnel *Wear eye protection throughout this activity* Procedure: The Effect of Solute Concentration on the Rate of Osmosis 1. Soak the 5 strips of dialysis tubing in a beaker of tap water for approximately 2 minutes. 2. Using masking tape, label the 5 beakers as follows (one label per beaker): 0% sucrose 20% sucrose 5% sucrose 40% sucrose 10% sucrose 3. Using transfer pipettes, the graduated cylinder, and distilled water, dilute the sucrose stock solution provided to produce 10 mL of the following three sucrose solutions: How to mix solution: 10 mL of 5% sucrose solution ____ mL of 40% sucrose solution + ____ mL of distilled water 10 mL of 10% sucrose solution ____ mL of 40% sucrose solution + ____ mL of distilled water 10 mL of 20% sucrose solution ____ mL of 40% sucrose solution + ____ mL of distilled water 10 mL of 40% sucrose solution 10 mL of 40% sucrose solution 4. Rub the dialysis tubing between your fingers to find an opening. Tie a tight knot near one end of the dialysis tubing. Repeat with the other pieces of tubing. 5. Using a graduated cylinder (and funnel, if necessary), pour 10 mL of the solutions you prepared in step 3 into corresponding dialysis tubes. (Rinse the graduated cylinder with tap water and shake dry between solutions.) 6. Fold over and twist the open end of each dialysis tube. Remove excess air from the tube, making sure that it remains limp, then tie each end in a knot. Dry the outside of the tubes with paper towel. Place each dialysis tube into the correspondingly labelled beaker. 7. Measure and record the mass of each dialysis tube. 8. Remove the dialysis tubes (being careful not to mix them up!) and fill each beaker with 200 mL of distilled water at room temperature. 9. Place the corresponding dialysis tube into each beaker and wait 20 minutes. 10. After 20 minutes, remove the dialysis tubes, blot them dry, and measure and record their masses (again, being careful not to mix them up!). 11. Use your recorded values to determine the percent mass change for each dialysis tube using the following equation: % mass change = (mafter - mbefore) ÷ mbefore x 100% Record the % mass change for each dialysis tube in the chart. 12. Discard the solutions and dialysis tubes according to your teacher's instructions. 13. Draw suitable graphs of your results. Reference: This lab was taken from pages 65-66 in: Biology 11 College Preparation (2003) Authors: Maurice Di Giuseppe, Douglas Fraser, Barry LeDrew, Jill Roberts Publisher: Nelson (Thomson Canada Ltd.) in Toronto, Ontario Answer the following questions. Include the answers to the Analysis & Evaluation questions in the Discussion & Conclusion section of the lab report. The lab design (Extension question) should be included as an Appendix in your lab report. Analysis Questions: a. Analyze your results for patterns. What can you conclude about how changes in solute concentration affect the rate of osmosis? b. Did all dialysis tubes increase in mass? If not, explain. c. Which dialysis tubes had the greatest increase in mass in Part 1? Why? d. Describe the five liquids in the dialysis tubes in Part 1 as hypotonic, hypertonic, or isotonic with respect to the distilled water in the beakers. Evaluation Questions: e. What was your hypothesis on how changes in solute concentration would affect the rate of osmosis? How accurate was your prediction? f. Describe any sources of error in Part 1 of the investigation. g. Suggest possible improvements to the procedure in Part 1. Extension Question: h. Design a procedure for determining the effect that a change in temperature has on the rate of osmosis. Include as many controls as possible, and list safety precautions.