Diffusion Labs
advertisement

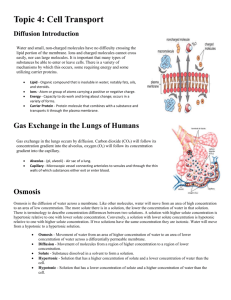
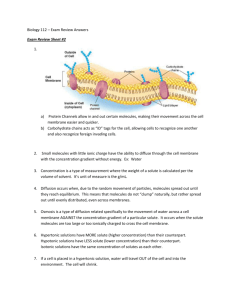
Diffusion and Cell Membranes - I Objectives 1. Define the following terms: solute, solvent, concentration gradient, osmotic pressure, and selectively permeable. 2. Define the following processes and identify the characteristics that distinguish them from one another: diffusion, osmosis, facilitated diffusion, and active transport. 3. Give three factors that affect the rate of diffusion and state whether they increase or decrease the rate. Introduction Cells maintain a constant internal environment, a process called homeostasis. In a constant environment enzymes and other cellular components can operate at optimum efficiency. The ability to selectively exchange materials with the environment is one component of the cell’s homeostatic mechanism. Ions and molecules, such as sugars, amino acids and nucleotides, must enter the cell, and the waste products of cellular processes must leave the cell. Regardless of the direction of movement, the common mediator of these processes is the cell membrane, or plasma membrane. The plasma membrane is a fluid, or mobile, mosaic of lipids and proteins. Ions and molecules cross the plasma membrane by a number of processes. Large particles are engulfed by the membrane, forming a vesicle or vacuole that can pass into (endocytosis) or out (exocytosis) of the cell. Some small, electrically neutral molecules diffuse through the spaces between the lipid molecules of the plasma membrane. Others bind to transport proteins embedded in the plasma membrane and transported into or out of the cell. Atoms, ions and molecules in solution are in constant motion and continuously collide with each other because of their kinetic energy. As the temperature is raised, the speed of movement of the molecules increases and they collide more frequently and with greater force. An observable consequence of this motion is Brownian motion, an erratic, vibratory motion of small particles suspended in water, which is caused by the collisions of water molecules with the particles. Diffusion also results from the kinetic energy of molecules. If a small crystal of a soluble substance is added to water, molecules of the substance break away from the crystal surface and enter solution. As a consequence of the collisions with water molecules, molecules of the substance move in a random pattern in the solution, but always away from the crystal, with some moving to the farthest reaches of the solution. This process continues until the molecules of the substance are evenly distributed throughout the water (the solvent). In a general sense, in any localized region of high concentration, the movement of molecules is, on average, away from the region of highest concentration and towards the region of lowest concentration. The gradual difference in concentration over the distance between the regions of high and low concentration is called the concentration gradient. The steeper the concentration gradient, the greater the rate of diffusion. The rate of diffusion is also directly proportional to the temperature and inversely proportional to the molecular weight of the diffusing molecules. In other words, the higher the temperature the faster the rate of diffusion, but larger molecules move slower than smaller molecules at the same temperature. Small, electrically neutral substances diffuse into and out of cells by passing through the spaces between the lipids of the plasma membrane or by dissolving in the lipids or proteins of the membrane. Substances that are large or electrically charged cannot pass through membranes. Membranes that block or inhibit the movement of molecules are called differentially permeable, or selectively permeable. Selective permeability explains the phenomenon of osmosis, the diffusion of water across a membrane under certain conditions. If two solutions containing different concentrations of a solute are separated by a selectively permeable membrane (permeable to water but not to the solute), water will move from the solution with low solute concentration to the solution of high solute concentration. The water flows in this direction because the solution with low solute concentration has a high water concentration and the solution with high solute concentration has a low water concentration. Thus, the water diffuses from a region of high water concentration to a region of low water concentration. Many ions and molecules important to cells are taken into cells by specific transport proteins found in cell membranes. Facilitated diffusion occurs when such a protein functions as a binding and entry port for the substrate. In essence, the protein functions as a pipeline for the specific substance. The direction of flow is always from high concentration to low concentration. The gradients are maintained because frequently the molecules are metabolically converted to other types of molecules once they enter the cell. For many other molecules and ions, favorable diffusion gradients do not exist. For example, sodium ions are present at higher concentrations outside mammalian cells than inside the cells, yet the net movement of sodium ions is from the inside to the outside of the cell. Likewise, potassium ions are found inside mammalian cells at significantly higher concentrations than outside the cell, but the net movement of potassium ions is from the outside to the inside of the cell. For such molecules and ions, cellular energy must be used to transport the molecules across the plasma membrane. Active transport occurs when transport proteins in the cell membrane bind with the substrate and use cellular energy to drive the “pumping” of the molecules into or out of the cell, against the concentration gradient. In today’s lab you will observe Brownian motion, osmosis, and diffusion in the solid, liquid and gaseous state, and investigate the parameters that affect the rate of diffusion of molecules. In next week’s lab, which is a continuation of your investigation of diffusion and the properties of cell membranes, you will model a semi-permeable membrane and investigate the behavior of different types of cells in hypotonic, hypertonic and isotonic solutions. Observing Brownian Motion The vibratory movement exhibited by small particles in suspension in a fluid was first observed by the Scottish botanist Robert Brown in 1827. Brown incorrectly concluded that living activity was the cause of this movement, but we now know that Brownian movement results from the collisions between water molecules and small particles (less than 10 micrometers in diameter) suspended in the water. To illustrate Brownian movement, place a drop of water on a microscope slide. Dip a dissecting needle into India ink and then touch the tip of the needle into the water drop. (India ink consists of small particles of carbon suspended in a fluid.) Add a coverslip and observe the slide with a high-power objective. Briefly record your impressions of the movement of the particles. If you gently warm the slide over a light bulb, what effect does this have on the movement of the particles? How do you account for any changes in motion you observe after heating the slide? Osmosis The rate of water movement in osmosis can be observed with an osmometer (see figure below). A starch solution in the thistle funnel is separated from the water in the beaker by a dialysis membrane that allows water to pass through but is impermeable to starch. (The starch solution has had food coloring added to it so that you can track any movement of the solution in the thistle funnel.) What do you expect will happen over time in this type of setup? Figure 1. A Simple Osmometer Early in the lab, measure the height of the column of fluid in the thistle funnel. At intervals of about 20 minutes during the lab, repeat the measurement. Record the time and height of the fluid column in the table below. Time Elapse Time Height of Fluid Column Describe what is happening to both the starch and water molecules in the osmometer. Over time do you expect that the rate of water movement will increase, decrease, or remain the same? Why? Diffusion in a Solid The solid we will use is agar, which forms a colloid (a gel-like matrix) when mixed with water, and is clear so you can see into it. Molecules can diffuse through the water-filled channels in the agar matrix. Your instructor may assign you to a group to carryout this experiment. Alternatively, your instructor may do this experiment as a demonstration and provide you with the data at the end of the lab period. o 1. Obtain o 6 agar plates for your group. Label one pair of plates 4 C, the second pair RT (for room temperature), and the third pair 37 C. 2. Take off the lids of the first pair of plates. Using a toothpick, place a small crystal of potassium permanganate (KMnO ) on 4 the agar surface of one plate, and a similar amount of methyl orange on the agar surface of the second plate. Be careful not to poke a hole in the agar surface. Replace the lid on each plate. 3. Repeat step 2 for the remaining pairs of plates, taking care to use similar sized KMnO crystals and methyl orange on each 4 pair of plates as used in step 2. o o 4. Place the 4 C plates o in a refrigerator, leave the RT plates in a safe spot on your lab bench, and the 37 C plates in an incubator set for 37 C. Record the time you start the experiment. 5. After about 1.5 hours, collect each pair of plates and measure the size of the colored ring around each crystal in millimeters. Record the radius of each ring in the appropriate box in table 1 below. Record the time you make the measurements and calculate the time, in minutes, the plates were sitting. Start Time End Time Length of time (min) Table 1. Radius of ring (mm) 4C Radius of ring (mm)Room Radius of ring (mm)37C Potassium permanganate Methyl Orange 6. Calculate the rate of diffusion for each molecule at each temperature using the procedure outlined below. a. Convert the radius from millimeters the micrometers by multiplying the radius in millimeters by 1000. b. Divide the resulting number by the number of minutes the plates were sitting. Record this result in the appropriate box of the following table. Table 2. Rate of diffusion (µm/min.) 4C Room 37C Potassium permanganate Methyl Orange Use this data to answer the following questions. 1. The molecules of which substance diffused more rapidly? 2. The molecular weight of KMnO is 158 and the molecular weight of methyl orange is 327. What relationship is there 4 between the molecular weight of the substance and the rate of diffusion? 3. What relationship is there between the rate of diffusion and temperature? What reason can you give to explain this relationship? Diffusion in a Liquid In this experiment we will be determining the rate of diffusion of KMnO in water at room temperature. We will then compare 4 this rate with the rate of diffusion of KMnO in agar at the same temperature (from the table above). 4 1. Place some room temperature water in a glass Petri dish and place the Petri dish over a thin, flat metric ruler. 2. Using tweezers place a crystal of KMnO directly over one of the millimeter lines of the ruler and record the time. 4 3. After 10 minutes, measure the distance the color has moved. Record the final time, length of time and distance moved in the table below. 4. Calculate the rate of diffusion of the KMnO using the procedure described above and record it in table 3 below. 4 Table 3. Start Time End Time Length of Time (min) Distance Moved (mm) Diffusion Rate (microns/second) Water Agar Transfer the appropriate data from table 2 above and use the data in table 3 to answer the following questions. 1. In which experiment is diffusion the fastest? 2. How can you explain this difference in speed? Diffusion in a Gas This experiment is an optional demonstration and will be done at the discretion of your instructor. It should be noted that the two chemicals involved have a real potential to be harmful and should be treated with extreme caution. The diagram below illustrates the experimental setup. 1. Remove the rubber stoppers from the end of the glass tube and simultaneously dip one of the cotton tipped applicator sticks into concentrated HCl and the other into concentrated NH OH. 4 2. Simultaneously reinsert the stoppers into the glass tube. 3. Look for the formation of a white ring inside the tube. This is NH Cl, a white salt formed when HCl and NH 4 3 meet. 4. Measure the distance each gas traveled and record the results in table 4 below and use the data to answer the following questions. Table 4. HCl NH3 Distance Traveled (mm) 1. The molecular weight of HCl is 36 and NH is 17. Which gas did (should) diffuse the fastest? 3 2. Calculate the following values: the ratio of the distances, the ratio of the molecular weights, and the ratio of the square roots of the molecular weights. Is the rate of diffusion directly or inversely proportional to the molecular weight or the square root of the molecular weight? Diffusion and Cell Membranes – II Objectives 1. Define the following terms: hypotonic, isotonic and hypertonic. 2. To determine if osmosis and diffusion both occur through a selectively permeable membrane. 3. To observe the effects of hypotonic, isotonic and hypertonic solutions on plant cells and animal cells. 4. Given any two solutions of differing osmotic potentials and separated by a selectively permeable membrane, state which solution is hypertonic and in which direction the net flow of water will occur. Introduction The term tonicity describes the relative concentration of solvent to solute in two solutions. A solution with the lower solute concentration is said to be hypotonic relative to the other solution. Conversely, the more concentrated solution is hypertonic relative to the first. If the solute concentrations of each solution are equal the solutions are isotonic with respect to each other. It is important to remember that these terms are relative terms, that is, the description of a solution as being hypertonic, hypotonic or isotonic depends on the solution it is being compared to. Traditionally, in biology, the cell is the frame of reference. An isotonic solution has the same solute concentration (and water concentration) as the cell; a hypertonic solution has a higher solute (and lower water) concentration than the cell; a hypotonic solution has a lower solute (and higher water) concentration than the cell. If a cell in a hypotonic solution (low solute concentration) is enclosed in a rigid box, for example a plant cell surrounded by the rigid cell wall, the increasing water pressure inside the cell would cause water to flow back out of the cell towards the area of lower pressure. Eventually, equilibrium would be reached when the flow of water into the cell, due to the concentration differences, equals the flow of water out of the cell, caused by pressure differences. The pressure at equilibrium is called the osmotic pressure. Since all cells contain molecules that cannot cross the plasma membrane, osmosis always occurs when cells are placed in dilute aqueous solutions. It is important, then, for cells to be able to regulate the flow of water into, and out of the cell, a process known as osmoregulation. In plant cells and bacterial cells, the cell wall prevents the cell from bursting by providing a rigid casing that helps regulate the osmotic pressure in the cell. In animals and many microorganisms, osmoregulatory organs or organelles are found. In animals the kidney adjusts the concentration of substances in the body fluids that bathe the cells. In microorganisms, like Paramecium, which live in freshwater, special organelles, called contractile vacuoles, accumulate and actively pump out water that flows into the cell by osmosis. In this week’s lab you will model a semi-permeable membrane and investigate tonicity by looking at the behavior of different types of cells in hypotonic, hypertonic and isotonic solutions. Diffusion Through a Selectively Permeable Membrane The plasma membrane of a cell is selectively permeable because it allows the diffusion of some substances and not others. Small, uncharged molecules diffuse freely across the plasma membrane, but charged molecules and large molecules cannot cross the membrane. The dialysis membrane used in this experiment simulates the activity of the plasma membrane. Procedure 1. Obtain a piece of dialysis tubing and make a tight knot in one end with thread. 2. Fill the bag with solution A, a simulated “liquid” meal containing 10% glucose, 1% starch, 0.5% egg albumin, and 1% sodium chloride. 3. Tie the top of the tube with thread while expelling as much air as possible. The bag should be limp (flaccid). 4. Rinse the outside of the dialysis tube with distilled water. 5. Place the dialysis tube in a culture dish and add enough solution B to cover it. Solution B contains 0.5% sodium sulfate dissolved in water. Let the dish stand undisturbed for about 1½ hours. 6. Based on the recipes for solutions A and B, fill in the “Before” columns of table 1. Use a + to represent the presence and a – to represent the absence of a substance. Table 1. Substance Inside Dialysis Tubing Before After Outside Dialysis Tubing Before After 1. Starch 2. Chloride Ion 3. Sulfate ion 4. Glucose 5. Albumin 7. After about 1½ hours, remove the dialysis tubing from the culture dish. Gently agitate the contents of the tubing and note any change in the tubing (Hint: is it more or less flaccid than when you started?) 8. Rinse the dialysis tubing with distilled water and carefully open the tubing. Empty the contents into a 100ml beaker. You can now test which ions and molecules crossed the membrane. 9. Obtain eight test tubes and prepare them as follows: a. Into each of the first four test tubes, place 10 drops of the solution from inside the dialysis tubing. Label these I-1 to I-4. b. Into the second set of four tubes, place 10 drops of the solution from outside the dialysis tubing. Label these O-1 to O-4. 10. Test for the presence of starch, albumin, glucose, sulfate ions, and chloride ions in the two sets of test tubes using the following test: a. To the first test tube of each set, add 3 drops of IKI to test for starch. A blue-black color indicates a positive result. b. To the second test tube of each set, add 1 drop of silver nitrate (AgNO ) to test for chloride ions. A 3 white precipitate indicates a positive result. c. To the third test tube of each set, add 3 drops of 1% barium chloride (BaCl ) to test for sulfate ions. A white 2 precipitate indicates a positive result. d. To test for the presence of glucose, dip a Clinistix into the fourth test tube of each set. Compare the results to the color chart on the side of the container. e. To test for the presence of albumin, dip an Albustix into the fourth test tube of each set. Compare the results with the color chart on the side of the container. 11. Record your results in the “After” columns of table 1. Use a + to represent a positive test and a – to represent a negative test. Use these results to answer the following questions. 1. At the start of the exercise, which solution (A or B) was hypertonic compared to the other (that is, which had the higher concentration of solutes)? 2. Which solution gained water in the course of the exercise (A or B)? 3. Which of the substances (starch, chloride ions, sulfate ions, glucose, albumin, and water) were able to pass through the membrane (in either direction)? 4. Which substance(s) moved out through the membrane? 5. Which substance(s) moved in through the membrane? 6. Why did each substance move in the direction it did? 7. By what process did the substances move across the membrane? 8. Why did some substances fail to pass through the membrane? 9. Would you expect all of the molecules of a diffusible substance to move across the membrane? Why? 10. Which of the following statements best describes the situation at equilibrium if you let the system stand for a long time? a. No molecules move across the membrane. b. All molecules cross the membrane equally often in either direction. c. Molecules to which the membrane is permeable cross equally often in either direction. d. Only water molecules cross the membrane equally often in either direction. e. Molecules to which the membrane is permeable move across the membrane from a region of high concentration to a region of low concentration. 11. Did water move across the membrane? What is your evidence? 12. What is misleading about trying to equate the results of this exercise with how the cell membrane regulates passage of material? 13. Dialysis membrane is permeable to iodine (IKI). What result would you expect to see if you put IKI in solution B at the start of the exercise? Osmosis and Tonicity—If set up… As you discovered last week, osmosis (the diffusion of water) occurs whenever two solutions of different solute concentration are separated by a selectively permeable membrane. The difference in solute concentration between the two solutions determines both the direction and rate of water flow. Water always diffuses from a hypotonic solution to a hypertonic solution; consequently, a cell placed in a hypotonic solution will gain water and a cell placed in a hypertonic solution will lose water. The next three experiments explore tonicity (the solute concentration of a solution) using potato strips, red blood cells and Elodea cells. Procedure: Potato strips 1. Using the provided cork borer cut 6 tubes of potato, each approximately 3 cm in length. Use a razor blade to cut the tubes to length; remove any skin from the ends of the tubes. 2. Label five test tubes 0, 0.1, 0.2, 0.3, 0.4, and 0.5. Place one potato tube to each test tube. 3. Fill the test tube labeled “0” with distilled water to cover the potato tube, and fill the remaining test tubes with sodium chloride solutions of the appropriate concentration to cover the potato tubes. 4. After at least 1 hour, observe the potato tubes for limpness (water loss) or stiffness (water gain) and answer the following questions. 1. In which tube(s) has the potato become limp? Why did the water diffuse out of the potato? How would you describe the relationship between the solution and the potato (use the correct scientific term)? 2. In which tube(s) has the potato become stiff? Why did the water diffuse into the potato? How would you describe the relationship between the solution and the potato (use the correct scientific term)? 3. Is there any tube(s) in which the potato appears to have neither gained nor lost water? How would you describe the relationship between the solution and the potato (use the correct scientific term)? 4. Based on these results, what is the approximate concentration of solutes in potato cells? The Effect of Solute Concentration on Red Blood Cells A solution of 0.9% sodium chloride is isotonic to red blood cells. In this solution red blood cells maintain their typical biconcave appearance. A solution greater than 0.9% NaCl is hypertonic to red blood cells. In such a solution, the cells shrivel, a process known as crenation. A solution of less than 0.9% NaCl is hypotonic to red blood cells. In such a solution, the red blood cells swell and burst, a process called hemolysis (lysis is the general term for cell bursting). These changes in appearance are visible in the light microscope. The lysis of a red blood cell can also be monitored in the test tube. A suspension of normal or crenated red blood cells will appear cloudy; as red blood cells lyse, however, the solution changes to a clear pink. In this exercise, you will test the effect of four solutions with differing NaCl concentrations on red blood cells. Procedure 1. Obtain four test tubes and mark each at 2 cm above the bottom with a wax pencil. Label the tubes 1,2, 3, and 4, respectively. 2. Fill to the mark with the appropriate saline (salt) solution: Tube 1 - 1% NaCl; Tube 2 10% NaCl; Tube 3- 0.5% NaCl; Tube 4- 0.05% NaCl. 3. Add one drop of blood to each tube. Immediately cover the tube with Parafilm and shake gently. Place the tube in front of your laboratory manual. Notice for tubes 1 and 2 that you cannot see the type on the page through the tube. However, in tubes 3 and 4 you should see the solution change from cloudy to clear. Record the time that is required for the type to appear. Time required for clearing: Tube 3 Tube 4 4. Make a slide with a drop from each tube. Examine the appearance of the cells microscopically using the 40X objective lens. 5. Record the appearance of the cells and the correct scientific term to describe it in Table 2. Table 2. Observations of Red Blood Cells Tube Solute Concentration Drawing of Cells (in test tube) 1 1% 2 10% 3 0.5% 4 0.05% Scientific Term Use the results to answer the following questions. 1. What is the approximate solute concentration in a red blood cell? 2. Solutions with a solute concentration greater than 1% are _______________ compared to a red blood cell. Solutions with a solute concentration less than 1% are _______________ compared to a red blood cell. Solutions with a solute concentration ______________1% are isotonic compared to a red blood cell. 3. What was the direction of water movement across the red blood cells in tube 1 (1% NaCl), tube 2 (10% NaCl), tube 3 (0.5% NaCl), and tube 4 (0.05% NaCl)? 4. By what process did the water move across the membrane? 5. Does this process require cellular energy? 6. Would you expect a red blood cell placed in a solution of 5% solute to be crenated, lysed,or normal? In a solution of 0.1% solute? 7. Why did the solutions in tube 3 and 4 become clear? 8. Which tube cleared more quickly (3 or 4)? Why? The Effect of Tonicity on Elodea Plant Cells Elodea is a plant that lives in fresh water. In such a hypotonic solution, the large, central vacuole of each Elodea cell gains water and exerts pressure (called turgor pressure), which compresses the cytoplasm and plasma membrane against the cell wall. In this situation the chloroplasts appear to be either evenly distributed throughout the cytoplasm or around the cell perimeter, with the central vacuole visible as a transparent region in the center, depending on the plane of the cell being observed. When an Elodea leaf is placed in 10% NaCl, a hypertonic solution, water moves out of the central vacuole and the cytoplasm into the surrounding solution. The cell volume is reduced, and the plasma membrane visibly pulls away from the cell wall, a process known as plasmolysis. In this situation, water concentration inside the cell is higher than water concentration outside the cell and the net flow of water is out of the cell. In this exercise, you will place an Elodea leaves in solutions of 0% and 10% solute. Fill out the first four columns of table 3 to predict the outcome of the experiment, then follow the procedure below. Procedure 1. Make a wet mount slide of an Elodea leaf in water, wait 5 minutes, then observe the leaf in the microscope. 2. Use the fine focus to view the top surface of the cells, then focus downward to view the central portion of the cells. 3. Draw a diagram of the cell’s appearance in the appropriate column of table 3. 4. Prepare a wet mount slide of an Elodea leaf in 10% NaCl, wait 2-3 minutes, then observe the leaf in the microscope. 5. Draw a diagram of the cell’s appearance in the appropriate box in table 3. Table 3. Effects of tonicity on Elodea cells Solute Water Concentration Concentration (in test tube) (in test tube) 0% Tonicity of Solution Direction of Water Movement Scientific Term Drawing of Cell 10% Answer the following questions. 1. Did your results support your hypotheses for the exercise with the Elodea cells? 2. What appearance would you expect an Elodea cell to have in a solution of 5% solute? Of 0.5% solute? The following questions will help you think about the differences in the responses of plant and animal cells with respect to tonicity. 1. Do plant cells ever lyse (burst) in hypotonic solutions? 2. Do animal cells (such as red blood cells) lyse in hypotonic solutions? 3. What structural difference in plant cells compared to animal cells accounts for this difference? Complete table 4 to compare the responses of red blood cells and plant cells to solutions of different solute concentration, or tonicity. Table 4. Summary of Observations Tonicity of Solution Red Blood Cell Plant Cell Physical Change Hypertonic Isotonic Hypotonic Scientific Term Physical Change Scientific Term