Osmosis Lab
advertisement

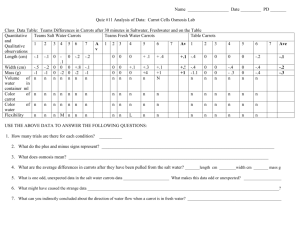
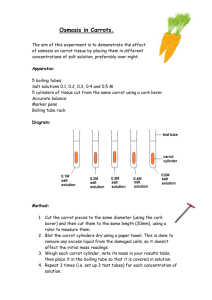
Osmosis Lab – [solution] vs. %change in mass in carrots Question: How does the [concentration] of a solution effect water movement within a carrot? Hypothesis: If the carrot is placed in increasing [salt] solution, then the carrot’s mass will (circle one) increase/ decrease/ stay the same because ____________________________________ _______________________________________________________________________________. Procedure: 1. Working in your assigned groups, obtain 4 beakers. Label them with a wax crayon as: control, 0%, 5%, 10%, 2. Obtain 4 carrot pieces 3. Use the balance to find the initial mass of each carrot. Record data in table. Remember, find the mass of the CARROT ONLY.. 4. Put designated carrot into each solution overnight. After 24 hours… 5. Mass each CARROT ONLY. Show math. Record in final mass. 6. Calculate the total change in mass and the percent change in mass. 7. Compile class data 8. Make a graph of class data on graph paper using proper graphing techniques. Data Table 1: Change in Mass of Carrots in Different [Salt] Solutions Control 0% salt 5% salt 10% salt Initial Mass (grams) Final Mass (grams) Total mass (+ or - value) % mass CLASS AVE. % mass = change [ ] = concentration % change in mass = total change in mass x 100 initial mass Carrots: End Texture Conclusion- RADdS Model – Be sure to include the answer to the 4questions below in your “S” section (Scientific explanation of results) 1. 2. 3. 4. What type of solution was control, 0% salt solution, 5% salt solution, 10% salt solution in relation to the carrot? Which carrot demonstrated the process of turgor (rigidity/increase in size/water gain)? Which carrot demonstrated the process of plasmolysis (shrinking/decrease in size/water loss)? What might happen if we take the carrots we just used and placed them in reverse beakers overnight?