Water Potential Lab: Potato Osmosis Experiment
advertisement

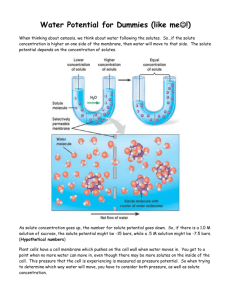
Water Potential based on Carolina Biological AP Biology Lab 1 As you have seen (refer to the Diffusion & Osmosis Lab) in animal cells movement of water into and out of a cell is influenced by the relative concentration of solute on either side of the cell membrane. In plants, the situation is complicated by the presence of a rigid or semi-rigid cell wall. As water enters the cell, the cell membrane is pressed against the cell wall; thus, a physical or hydrostatic pressure develops that opposes the entrance of additional water. When the pressure inside the cell becomes great enough, no additional water can accumulate in the cell, even though the cell still has a higher solute concentration than does pure water. Thus movement of water through plant tissue cannot be predicted simply by knowing the relative solute concentrations on either side of the plant cell membrane. It is necessary to also account for any differences in pressure between the two sides. The concept of water potential is used to combine the differences in solute concentration and pressure to predict the direction in which water will diffuse through living plant tissues. Traditionally, water potential is expressed in bars which is approximately equal to one atmosphere. Water potential is abbreviated by the Greek letter psi (�) and has two major components: solute potential (�p), which is dependent on solute concentration and pressure potential (�p), which results from the exertion of pressure (either positive or negative) on a solution. Mathematically: Water potential = Pressure Potential + Solute Potential � = �p + �s The water potential of pure water is zero. If a substance is dissolved in water, the water potential drops below zero since there is fewer water molecules available for osmosis. Notice that the solute potential is always zero or negative. Pressure potential may be positive, negative or zero. Although water diffuses in all directions, the net movement of water will be from an area of higher water potential to an area of lower water potential. For example, if a potato cell is suspended in pure water in a beaker (open to the atmosphere) �p = 0. If we assign a value of -9 for �s, then the initial conditions can be described as follows: Pure water: � = �p + �s or �= 0 + 0 = 0 Potato Cell: � = �p + �s or � = 0 + (-9) = -9 Since -9 is less than 0, water will diffuse into the cell. At equilibrium: Pure water: � = �p + �s or �= 0 + 0 = 0 Potato Cell: � = �p + �s or � = 9 + (-9) = 0 There will be no net movement of water into or out of the cell. Water Potential based on Carolina Biological AP Biology Lab 1 Suppose we added solute to the water in the beaker to produce the following conditions: Water + Solute: � = �p + �s or � = 0 + (-15) = -15 � = �p + �s or � = 0 + (-9) = -9 Potato Cell: Since -15 is less than -9, water will diffuse out of the cell. If this continues, the cell will shrink and the cell membrane may pull away from the cell wall, a condition known as plasmolysis. This often injures or kills the cell. If we know the molarity of a sucrose solution that will produce equilibrium between the solution and the contents of the cell, we can determine the solute potential by the following: �s = -iCRT i = ionization constant (for sucrose the value is 1) C = molar concentration R = pressure constant of 0.0831 liter bar/mole K T = temperature of solution in kelvins (K = oC + 273) Practice: Your solutions of sucrose for the osmosis lab were 0.2, 0.4, 0.6, 0.8 and 1.0 M. Room temperature was 22 oC. Calculate the solute potential (�s ) for each of your solutions. Show your equations. 0.2M ___________________________________________________________________ 0.4M ____________________________________________________________________ 0.6M ____________________________________________________________________ 0.8M ____________________________________________________________________ 1.0M ____________________________________________________________________ If these solution are in open beakers, what is the water potential for each? _______________ ___________________________________________________________________________ Materials: plastic cups or beakers, distilled water, sucrose, balance, cork borer, potato cores, ruler, scalpel, plastic wrap, paper towels. Procedure: 1. Make 100 mL of the sucrose solution assigned to your group. Show the math used to determine the amount of sucrose needed. _______________________________________________________________________ 2. Use a cork borer to cut four cylinders of potato. Trim both ends of each cylinder to remove the skin. Cut each cylinder into 3 cm long sections. Water Potential based on Carolina Biological AP Biology Lab 1 3. Keep potato bores wrapped in plastic wrap while not in use. (why?) 4. Find the total mass of all the potato sections and record as the initial mass. 5. Place all of the potato sections in a labeled cup. Pour 100 mL of the assigned sucrose solution into the cup. 6. Cover the cup with plastic wrap and allow it to set overnight. 7. Record the room temperature (provided by teacher). 8. Remove the potato sections from the sucrose solution. Gently blot them on paper towels to remove excess solution from surface. Keep them wrapped in plastic wrap while not in use. 9. Find the mass of all the potato sections and record as the final mass. 10. Record the initial mass and final mass from all the groups. ANALYSIS 1. Calculate the change in mass and the percent change in mass for each concentration. If more than one group had the same concentration, find the average percent change in mass. Record. 2. Graph the percent change in mass for each solution. 3. Find the point on the graph where the line crosses the 0 line (x-axis). Record the value of the y-axis. What is significant about this value? 4. Using the information from #3, calculate the solute potential at equilibrium. Show your calculations. �s = -iCRT 5. Using the information from #4 and the formula for water potential calculate a. The water potential of the solution at equilibrium b. The water potential of the potato cells at equilibrium CONCLUSION At a minimum, include an explanation of the direction of water movement in each solution. Justify your response mathematically. Explain why the ionization constant for sucrose is 1.0. What would the ionization constant for salt (NaCl) be? Explain. What are some possible sources of error? Why is this lab not applicable if we were using a section of beef instead of potato?