RECORDING DATA
advertisement

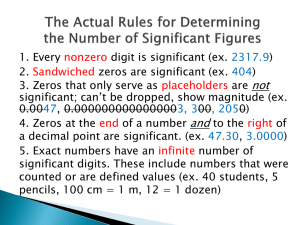
Reading: Recording Data Revised 9/10/13 RECORDING DATA Laboratory data should be recorded in a clear and concise way. Data and observations should be well organized and easy to read. The data recorded in a chemistry laboratory can be categorized as either qualitative or quantitative. The significant detail required in recording of both types of data is outlined in the following pages. Qualitative Data Qualitative data deals with chemical or physical qualities. These qualities typically take the form of visual observations. An experimenter must always note the appearance of the chemical substances with which s/he is working. The following observations should always be taken: • Color and color depth (hue) of all substances • Clarity or opaqueness of a liquid • Level of crystallinity of a solid reagent or product (ranging from large shiny crystals to dull powders). • Visual evidence of the occurrence of a reaction, such as precipitation and gas formation. (And the rate of gas formation should be indicated as slow, moderate, or vigorous.) • Physical sensations of hot or cold. For example, the warming of a flask during a reaction. • • When using an analytical instrument, record the name of the manufacturer, instrument number etc. When working with a partner or a group, record the names of the other students. This list is by no means complete: a good experimenter must be observant! Quantitative Data: Measurements Quantitative data deals with chemical or physical quantities or measurements. Measurements consist of two parts: numbers and units. Units indicate the type of measurement being taken (e.g. the presence of °C indicates the measurement of a temperature using Celsius degree units). All measurements must include units and all measuring devices should be identified with manufacturer names and serial numbers (e.g. Mettler Balance #20). Numbers indicate the amount of a particular unit present. The amount of figures recorded in a number (the significant figures) are dictated by an inherent uncertainty in measurements. 1 Reading: Recording Data Revised 9/10/13 Significant Figures All experimental measurements have some degree of error. Therefore, data should be recorded in a systematic way to unambiguously convey the degree of uncertainty to the reader. Significant figures are the certain and uncertain digits recorded in a measurement. The certain digits are transcribed directly from the markings on the measuring device. The uncertain digit, the last figure of the measurement, is estimated from between the last two markings. Only 1 uncertain digit should be recorded for each measurement. 18 18 17 17 17.2 or 17.3 oC 16 17.23 or 17.24 oC 16 B A Figure 1: Thermometers A and B show different sensitivity in the degree markings. The accuracy of the two thermometers in Figure 1 is conveyed by the number of digits used to record the temperature. The appropriate one-degree increment markings on thermometer A are recorded as the certain digits in the temperature measurement. To complete measurement the last uncertain digit must be estimated to a tenth of a degree. This last digit is "uncertain" because personal judgment is involved in determining this number. The temperature may be recorded as 17.2 °C or 17.3 °C and contains three significant figures (3 digits). A temperature recording of 17.25 °C from thermometer A would be incorrect as four significant figures indicate a thermometer with more markings. Temperatures can be measured with thermometer B to a tenth of a degree with certainty and estimated to a hundredth of a degree. The temperature is recorded as 17.23 °C or 17.24 °C and 2 Reading: Recording Data Revised 9/10/13 contains four significant figures. A temperature reading of only 17.2 °C from thermometer B would be incorrect, as three significant figures indicate a thermometer with fewer markings. While the sensitivity of measuring devices containing gradation markings (like the thermometers above) is readily apparent, the accuracy of a volumetric flask or pipet containing only one calibration line may not be as obvious. However, closer examination of the manufacturer information on the glassware indicates the accuracy of the volume measurements (Figure 2). Fill line: When the tip of the meniscus touches this line the volumetric flask is to contain (TC) 25 + 0.05 mL of liquid at 20oC. 25 mL Figure 2. Indication of error and sensitivity provided on volumetric glassware. Masses from digital scales should be transcribed exactly as they appear. The last digit on the right is uncertain, it is “estimated” by the scale. Significant Figure Rules The use of significant figures insures an experimenter is correctly communicating the accuracy of a measurement when recording data. Furthermore, understanding significant figure rules enables the interpretation of the accuracy of another experimenter’s measurements. The following digits are always significant: nonzeros, captive zeros (zeros between nonzeros), and trailing zeros (zeros to the right of nonzeros when a decimal point is present). A number 3 Reading: Recording Data Revised 9/10/13 containing trailing zeros should be written in scientific notation so the measurement’s accuracy is communicated without ambiguity. Leading zeros (zeros to the left of nonzeros) are never significant. Exact numbers (numbers from definitions or number of trials) are infinitely significant. Examples: All nonzeros are significant: 763 torr contains 3 significant figures. Captive zeros are significant, leading zeros are not: 0.0409 grams contains 3 significant figures. The first two zeros on the left are “leading”, the zero between the four and nine is “captive”. Trailing zeros can be ambiguous, so a decimal point must be used to indicate which digits are significant: 200 moles is ambiguous. If 4 significant figures are required for accuracy, the measurement should be expressed as 200.0 mol or 2.000 x 102 mol. If 2 significant figures are required, then 2.0 x 102 mol is correct. Exact numbers are infinitely significant. To calculate the average of 4 concentrations, the concentrations are summed and then divided by an infinitely significant 4 (4.000000……). 0.432 M + 0.428 M + 0.437 M + 0.424 M = 0.430 M 4 To convert from meters to centimeters a measurement is multiplied by a ratio containing an infinitely significant 100 cm (100.00000000000…….) and 1 m (1.000000000….). 6.90 x 10-2 m x 100 cm = 6.90 cm 1m Note that exact numbers have no effect on the number of significant figures in the answer. Calculations with Significant Figures When addition or subtraction is performed, the calculated result has the same number of decimal places as the measurement with the smallest number of decimal places. 207.65 g – 6.985 g = 200.67 g When multiplication or division is performed, the calculated result has the same number of significant figures as the measurement with the smallest number of significant figures. 4 Reading: Recording Data Revised 9/10/13 25.00 mL x 0.921 g/mL = 23.0 g For logarithms (log x = y ó 10y = x), the number of digits in x is equal to the decimal places of y. For example, log 23 = 1.36 and 101.89 = 78. The same rules can be applied to natural logarithms (ln x = y ó ey = x). For example, ln 23 = 3.14 and e1.89 = 6.6. When recording numerical data, use units and significant figures to express the scope and sensitivity of the measurement. General Rules about significant figures and some basic laboratory equipment: • Buret volumes should be recorded to the 2nd decimal place (watch for round-offs). • Volumetric pipet and flask volumes < 100 mL should be recorded to the 2nd decimal place. • Volumetric pipet and flask volumes > 100 mL should be recorded to the 1st decimal place. • Graduated cylinder and temperature readings depend on the sensitivity of the device used. • All digits should be recorded from a digital readout. 5