Data Recording and Significant Figures
advertisement

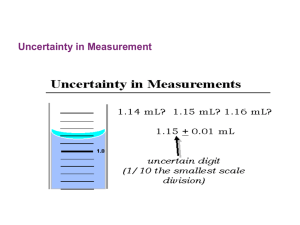
Data Recording and Significant Figures Data Recording and Significant Figures Accuracy and Precision Accuracy • How close a measurement is to the accepted value Precision • repeatability in measurements. • The number of decimal places. For example: 0.1g has less precision than 0.100g. Recording measurements off of equipment. estimate one more digit than the equipment provides. Recording measurements continued Exception: with electronic equipment, the user is rounding off digits rather than adding them. For example, 2.3456g will be rounded to 2.35g. Round to nearest .001g. Using uncertainty in measurements The estimated digit used when recording data can be written with an uncertainty notation, ± in the measurement. Example; a graduated cylinder that measures to the nearest ml is used for volume measurements. A student measures 3 different volumes using the same cylinder: 15.5ml, 15.8ml, 15.2ml in an experiment. How would one record these measurements using uncertainty? Significant Figures What are significant figures? • They are digits in measurements that were actually measured or estimated in some way. • Numbers that are not measurements are not considered to be significant figures. Examples are 100 in percent equations, pi, and constants used in scientific equations Rules for using zeros. Rule 1: Leading zeros are not significant. These are zeros which precede digits in decimal numbers. • Examples: 0.045g., 0.23 Rule 2: Captive zeros are significant. These are any zeros that are in between non zero numbers. • Example 2,013, 0.0101, 100.01 Use of zeros continued Rule 3: Trailing zeros are not significant. These are zeros at the end of large numbers with no decimal point. • Examples: 100, 10, 2,340 35,000 Scientific notation is used to remove the trailing zeros. Example: 35,000 becomes 3.50 x 104 Rules for rounding off measurements Rule 1: when reducing the number of digits, look at the first digit that must be eliminated. • If it ends in a number greater than 5 round up. • If it ends in a number less than 5 round down. • If it ends exactly in 5, round to the nearest even number. Rules for rounding in calculations Addition and subtraction • Round off the final answer to the same number of decimal places as the measurement with the fewest decimal places. • Examples. 2.34g + 2.4g + 2.35g=7.09g this should be rounded to 7.1g Rounding continued Multiplication and division • The final answer has the same number of significant figures as the measurement with the fewest significant figures. • Example 150.ml x 2.0 x 4.14 = 1242 this must be rounded off to : 1.2x 103