Scientific Notation with Negative Powers of 10
advertisement
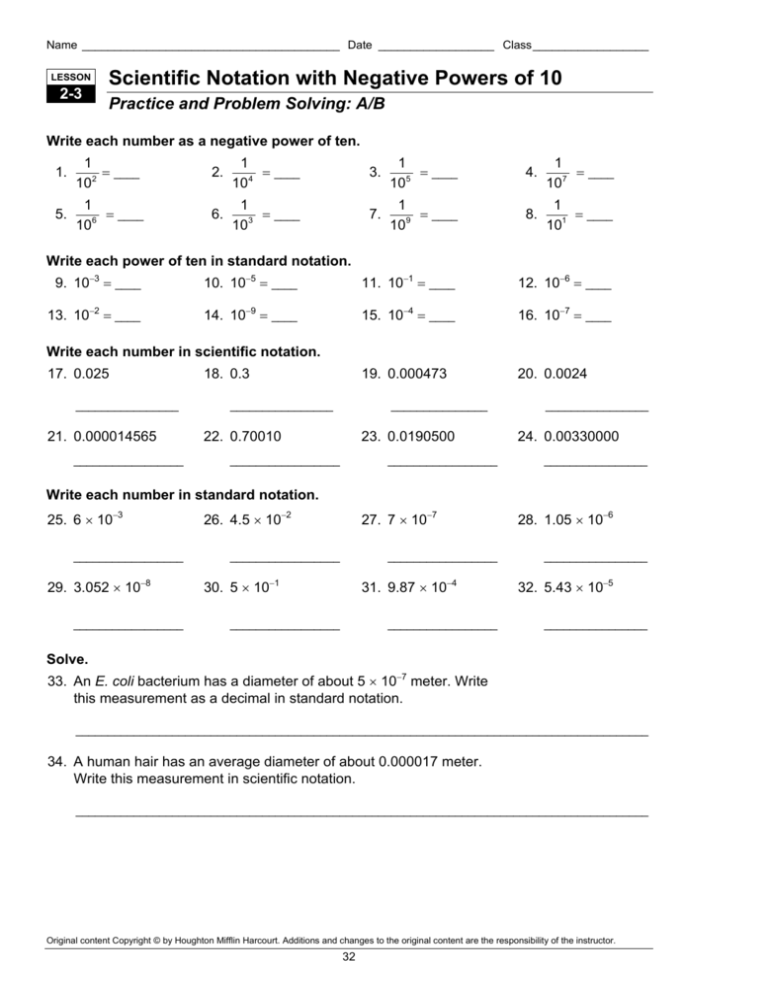
Name ________________________________________ Date __________________ Class __________________ LESSON 2-3 Scientific Notation with Negative Powers of 10 Practice and Problem Solving: A/B Write each number as a negative power of ten. 1. 1 = ____ 102 2. 1 = ____ 104 3. 1 = ____ 105 4. 1 = ____ 107 5. 1 = ____ 106 6. 1 = ____ 103 7. 1 = ____ 109 8. 1 = ____ 101 Write each power of ten in standard notation. 9. 10−3 = ____ 10. 10−5 = ____ 11. 10−1 = ____ 12. 10−6 = ____ 13. 10−2 = ____ 14. 10−9 = ____ 15. 10−4 = ____ 16. 10−7 = ____ 19. 0.000473 20. 0.0024 Write each number in scientific notation. 17. 0.025 ________________ 21. 0.000014565 _________________ 18. 0.3 ________________ _______________ 22. 0.70010 23. 0.0190500 _________________ _________________ ________________ 24. 0.00330000 ________________ Write each number in standard notation. 25. 6 × 10−3 _________________ 29. 3.052 × 10−8 _________________ 26. 4.5 × 10−2 27. 7 × 10−7 _________________ _________________ 30. 5 × 10−1 31. 9.87 × 10−4 _________________ _________________ 28. 1.05 × 10−6 ________________ 32. 5.43 × 10−5 ________________ Solve. 33. An E. coli bacterium has a diameter of about 5 × 10−7 meter. Write this measurement as a decimal in standard notation. _________________________________________________________________________________________ 34. A human hair has an average diameter of about 0.000017 meter. Write this measurement in scientific notation. _________________________________________________________________________________________ Original content Copyright © by Houghton Mifflin Harcourt. Additions and changes to the original content are the responsibility of the instructor. 32 30. 0.5 Success for English Learners 1. 3.28 × 10 > 3.28 × 10 because 3.28 × 105 = 328,000 and 3.28 × 103 = 3,280. 31. 0.000987 2. A car; 3 × 106=3,000,000 g, or 3,000 kg, and a car would weigh many kilograms but a hair would not. 34. 1.7 × 10−5 m 5 3. 1.86 × 10 3 32. 0.0000543 33. 0.0000005 m Practice and Problem Solving: C 5 1. 0.0052; 0.0000052; > 4. 4,567.89 2. 0.000005; 0.000025; < 3. 3; 0.1; > LESSON 2-3 4. 0.00502; 0.000205; > Practice and Problem Solving: A/B 1. 10 5. 1.2 × 10−3; 4.5 × 10−1 −2 6. 3.2 × 10−4; 2.3 × 10−6 2. 10−4 7. 2.35 × 10−6, 3.25 × 10−6, 5.32 × 10−6, 2.35 × 10−5, 3.25 × 10−5, 5.32 × 10−5 3. 10−5 4. 10−7 5. 10 8. 0 × 100, 1 × 10−1, 5 × 10−1,1 × 100, 5 × 100 −6 6. 10−3 9. 1 × 10−3 m = 1 mm; 1 ×10−1 cm = 1 mm; equal, so false 7. 10−9 10. 7 × 10−1 cm = 7 mm; 7 × 10−3 m = 7 mm; equal, so false 8. 10−1 9. 0.001 11. 3.5 × 10−1 cm = 3.5 mm; 3.5 × 10−3 m = 3.5 mm; equal, so true 10. 0.00001 11. 0.1 12. 9 × 10−1 mm; 9 × 10−4 m = 9 × 10−1 mm; equal, so true 12. 0.000001 13. 0.01 13. 9 ×10−3 liters = 0.009 liters; 3 × 10−5 liters = 0.00003; 0.009 ÷ 0.00003 = 300 drops 14. 0.000000001 15. 0.0001 19. 4.73 × 10−4 14. 0.00025 meters = 2.5 ×10−4; 0.000125 meters = 1.25 × 10−4; area = length × width = (2.5 × 10−4) × (1.25 × 10−4) = 3.125 × 10−8 square meters; to two significant digits, the answer is 3.1 × 10−8 square meters. 20. 2.4 × 10−3 Practice and Problem Solving: D 16. 0.0000001 17. 2.5 × 10−2 18. 3 × 10−1 21. 1.4565 × 10−5 22. 7.001 × 10 −1 23. 1.905 × 10−2 24. 3.3 × 10 −3 25. 0.006 26. 0.045 27. 0.0000007 28. 0.00000105 1. 1 100 2. 1 100,000 3. 1 10,000 4. 1 1,000 29. 0.00000003052 Original content Copyright © by Houghton Mifflin Harcourt. Additions and changes to the original content are the responsibility of the instructor. 329