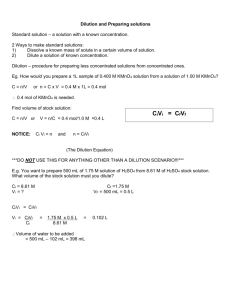
Unit IV
advertisement

MICRB 202: Introductory Microbiology Lab Unit IV: Estimating Cell Density of Laboratory Cultures 1 Unit IV: Estimating Cell Density of Laboratory Cultures Activities: 7.1 Viable Plate Counts (Ex 11) 7.2 Estimating Cell Density by Turbidity (Ex 12) Appendix 4.1: Performing Quantitative Dilutions p 1 p 4 p 6 Introductions: In many situations microbiologist need to determine the number of viable (live) organisms in a sample population. The enumeration of cells in a sample can be accomplished by a number of methods. Some of the most commonly used are: Viable (standard) Plate Counts, Turbidity, and Total Direct Counts. Viable plate counts rely on quantitative dilution and cultivation of the sample on agar media. The colony count on a plate is related back to the volume of initial culture used as inoculum, and reported as colony forming units (CFU) per milliliter (/mL). This assay only determines the density of viable and culturable bacteria in the sample. Turbidity, as we saw in Exercise 12, is an easy way to assess cell density of a culture; however, its values must be calibrated to some other measure of cell density. The optical distortion causing a turbid culture is due to both live and dead bacteria; so turbidity values may at times be unreliable when intending to relate them to live cell abundance. Microscopy can be used for direct counting of bacterial cells (live and dead) in a known volume of sample. The simplest approach uses a phase contrast-microscopy and a special counting chamber. The number of cells counted within a known area of the chamber is related back to cell density. Other microscopy methods for directly counting bacteria use special stains to enhance visualization of cells; some stains can even differentiate between live (metabolizing) and dead cells. Often these stains are fluorescent and require a special fluorescent microscope. We will use the later approach in future lab unit on water quality, but for the next few periods we’ll determine cell density by viable plate counts and use this value to calibrate turbidity measurements. 7.1 Viable Plate Counts: (Ex 11) In this method a sample or appropriate dilutions (usually 0.1–1 ml) of it are plated on or in an agar medium which will support the growth of some, if not all the bacteria in the sample. The method assumes that, after the incubation period, one cell gives rise to a homogeneous population or colony. In reality, it is possible that some of the colonies arise not from one individual cell but from chains and clumps that were not broken apart before plating. Because there is no certainty that all colonies come from a single cell, the count of colonies is reported as colony forming units (CFU’s) rather than number of viable cells. The method produces a good measure of the viable cells in the population and it is much more sensitive than the turbidity method described below. On the negative side, the Viable Plate Count Method is time consuming, labor intensive, and consumes large amounts of media and supplies. MICRB 202: Introductory Microbiology Lab Unit IV: Estimating Cell Density of Laboratory Cultures 2 A sample may contain more bacteria than can be counted as colony-forming units on one plate. In order to obtain plates with countable numbers of colonies, between 30 and 300 per plate (this number range produce the most statistically reliable results), a series of dilutions are made of the sample and plated. This assures that one of the plates will be in the right range to count colonies, and the dilution factor for that diluted sample can be used to calculate back to the CFU/mL in the original sample. Plating the samples may be done in one of two ways: (a) pour plates: the sample is mixed with molten agar so colonies grow within the agar medium; (b) spread plates: a small sample (usually 0.1 ml) is spread over the surface with a sterile spreader, so colonies grow on the surface of the agar. We will use the later today (more on the method below). EXERCISE 11: Viable (standard) Plate Count: Materials: TSB culture of your assigned bacterium 7x tubes with 4.5 mL sterile water 4x 1 mL disposable pipettes blue pipette pump 6x nutrient agar plates 250 mL beaker with 75% ethanol Glass spreading rod (“hockey stick”) Procedures: Serial Dilution of Culture: 1. Label seven tubes containing 4.5 ml sterile water with a ten-fold dilution value from 101 to 107. 2. Make sure that the cells in the 24h stock culture are suspended homogeneously, with no pellet remaining on the tube bottom. Do this by tapping the bottom of the tube with your index finger while holding the tube near the top with the other hand (be very careful not to spill the tube contents). Alternatively, pipette the culture up and down a few times with a 1 mL pipette. 3. Pipette 0.5 ml of your bacterium culture into the first dilution tube to make the 101 dilution. MIX VERY WELL by pipetting up and down. This not only mixes your dilution, but also allows the use of the same pipette in the next transfer. 4. Using the same pipette, continue making dilutions up to 107. Remember to mix very well by pipetting up and down before making each subsequent dilution. You are now ready to spread your plates. MICRB 202: Introductory Microbiology Lab Unit IV: Estimating Cell Density of Laboratory Cultures 3 Spread Plate Technique: This is an aseptic technique used to spread a volume of liquid bacterial suspension onto the agar surface of a pre-poured plate using a “hockey-stick” shaped sterile glass spreading rod. Usually, an amount between 0.1 ml – 1 ml of liquid culture is spread. By convention, when a 0.1 ml of a dilution is spread this counts as an additional 10 fold dilution from the original source. When a 1 ml of a dilution is spread this is still counted as the original dilution (this is because 0.1 ml is 1/10 of a ml ). How is the technique performed? The well mixed bacterial solution is aseptically pipetted onto the agar surface and spread evenly on the agar surface with the sterile glass spreading rod. The glass rod is sterilized by dipping it in a beaker with 75% ethanol and then the alcohol is removed by briefly flaming the glass rod to ignite and burn-off the ethanol. Do remember that after flaming the rod will be hot and it should be allowed to cool down before using it to spread the bacteria. The technique will be demonstrated by your instructor. 5. Label the bottom of six nutrient agar plates from 102 mL to 108 mL. These values represent the product of the dilution factor times a 0.1 mL inoculum (e.g. 0.1 mL of the 10-2 dilution tube will inoculate your 10-3 mL plate). Also write your initials and initials of the bacterium you work with. 6. Start at the highest dilution and with a new pipette (you will keep using this one from now on). Pipette 0.1 ml of the 10-7 dilution onto the plate labeled 10-8 mL. (work fast and move to step 7 right away) 7. Spread the inoculum with alcohol sterilized glass spreader. 8. Repeat steps 6 and 7 for the other dilution, progress from high dilution to low dilution tubes. 9. When all plates have been spread, invert them and incubate for 48 hours at 37 C. <<NEXT PERIOD>> Analysis and Computation: 10. Examine your plates and count the number of colonies on the plates that have between 30-300 colonies. If all of your plates have too many or two few talk to your instructor. 11. Proceed to calculate the number of colony forming units (CFUs) per ml that were in the original 24h bacterial culture using only plates with between 30 and 300 CFUs. The original sample value will be the CFUs divided by the volume of original culture used as inoculum that is written on the plate. For example, if you inoculated the plate with 0.1 mL of the 10-5 dilution, then this means you had inoculated the plate with 10-6 mL of the original (undiluted) culture. If 50 CFUs were counted on this plate, labeled 10-6 mL, then the original culture was 50 CFUs / 10-6 mL = 5 x 107 CFU/mL. Do the same calculations for each of the plates that can be counted. Calculate the average (the numbers should be relatively close) and report the average as the CFU’s for the original culture. MICRB 202: Introductory Microbiology Lab Unit IV: Estimating Cell Density of Laboratory Cultures 4 7.2 Estimating Cell Density by Turbidity: (Ex 12) You may have notice that growth of bacteria in liquid culture turns the broth cloudy, or turbid. This turbidity is due to the presence of tens of millions to billions of bacteria in the culture. The spectrophotometer is a device that can be used to quickly measure turbidity, which can be quickly converted to an estimate cell density using a calibration factor specific for the bacterium and media. A spectrophotometer quantifies the amount of light from a known internal source that passes, or is transmitted, through a sample to a detector (photocell). Without any sample in place, the instrument will read 100% transmittance, or zero absorbance of light. When a test tube containing a suspension of bacterial cells is inserted in the instrument, some of the source light will be absorbed and scattered as it passes through the cells. The reduction in the % transmittance will be proportionate to the cell density; more cells will scatter and absorb more light, so less transmittance. However, one should know that the relationship between transmittance and absorbance by the sample is not linear. A sample’s ability to absorb light is referred to as its optical density (O.D.), which equates to % transmittance according to the following function: O.D. = 2 – log of percent transmittance We will make readings off the spectrophotometer in units of absorbance, i.e. optical density. The most reliable values for bacterial culture turbidity are between 0.1 and 0.8 absorbance. A minimum number of cells (about 107 cells/mL) are required for an accurate reading at the lower end. The is a loss in linearity between culture density and absorbance at values greater than 0.8, due to source light reflection by the very large mass of cells. Furthermore, at these cell densities some of the cells will effectively shield others cells from the light. Both reflection and self shading will result in an underestimate the actual cell count. It is important to remember that the turbidity method will only produce a (rough) estimate of viable cells per ml of culture because both living and dead cells are measured. However, the method is useful to produce a quick estimate of the cell population as long as a standard curve is available to calibrate turbidity to cell numbers. A standard curve is made by plotting the Absorbance values against the CFUs obtained when the same sample is evaluated by the viable plate count method . This graph can be used to rapidly estimate a bacterial population in a new sample of the same species. Standard curves are very important for research concerning bacterial physiological properties, setting up immunological and molecular assays, etc. In all these lab procedures, known amounts of cells are required to standardize assay conditions; turbidity allows us to make real-time estimates. EXERCISE 12: Turbidity Assay: Materials: 6 Tubes containing 3 ml of tryptic soy broth (TSB) 24 h old culture of E. coli 5 ml pipettes green pipette pump MICRB 202: Introductory Microbiology Lab Unit IV: Estimating Cell Density of Laboratory Cultures 5 Spectronic 20 Kim Wipes Procedures: Turbidity of Diluted Culture: 1) Prepare the spectrophotometers as instructed previously (see Appendix 3.1 from last week). Again use a tube with 3 mL TSB to zero the instrument. 2) While waiting for the spectrophotometer to warm up, prepare a series of two-fold dilutions (1/2, 1/4, 1/8, 1/16, 1/32) from the 24 h culture of E. coli used in Exercise 14. Proceed as follows: a. Label 5 tubes containing 3 ml TSB with the dilution value (1/2, 1/4, 1/8, 1/16, 1/32). b. Make sure that the cells in the 24h stock culture are suspended homogeneously, with no pellet remaining on the tube bottom. Do this by tapping the bottom of the tube with your index finger while holding the tube near the top with the other hand (be very careful not to spill the tube contents). Alternatively, pipette the culture up and down a few times with a 5 mL pipette. c. Transfer 3 ml of the culture suspension into the tube labeled ½ and MIX WELL. This is the first two-fold dilution of your stock solution. d. Transfer 3 ml of the ½ dilution into the tube labeled ¼ and mix well. What is the bacterium content of this dilution with respect to the stock culture? e. Keep repeating this serial procedure to make 1/ 8, 1/ 16, and 1/ 32 dilutions of the stock culture 3) Read the Absorbance of each of the 5 dilutions made and record your results in the lab notebook. << NEXT WEEK>> Standardize Turbidity to Viable Plate Count: 4) Using the estimate of CFU/mL in the original culture, calculate the CFU/mL for each dilution you measured turbidity on. Enter these data in an Excel spreadsheet for plotting. 5) Plot CFU/mL (y-axis) versus turbidity (x – axis); label axes well, including units. Fit a linear regression to your data and place the equation on your plot. Attach this graph in your lab notebook, and write a proper figure legend underneath the plot. 6) Compare your result with a lab colleague working with a different bacterium. Was the turbidity to CFU/mL relationship similar? Think how you would test this statistically. MICRB 202: Introductory Microbiology Lab Unit IV: Estimating Cell Density of Laboratory Cultures 6 Appendix 4.1 Performing Quantitative Dilutions What does it mean to do a quantitative dilution? It simply means to thin down a culture by mixing it with another liquid, called the diluent. The actual dilution is most commonly calculated by dividing the initial sample volume by the total volume obtained after mixing with the diluent. Why do we quantitative dilute? There are many reasons, including diluting a sample for analyzes or diluting a stock solution to working solutions. For example one may need to prepare a small amount of a solution at a very low concentration. Say, you need to prepare 1ml of 0.05% NaCl w/v; this will require you to weight 0.0005 g of NaCl which is not possible. A way to get around it is to make a stock solution of say 0.1% NaCl and dilute a portion of it to make your 0.05% dilution. In microbiology we may have a culture containing millions of bacteria per ml of culture. We cannot count millions but we can count a small sample of the population and relate back to the original population. Two things are needed to accomplish this: a) Need to get a sample of the population that is small enough but manageable b) Need to keep track of what size is this fraction of the population so you can figure out what the total is. These are accomplished by using the appropriate quantitative dilution techniques Dilution = volume of sample / total volume (i.e. sample + diluent) Division of the numerator (volume of original sample) by the denominator (total final volume of the dilution) will give you the amount of material (sample) per milliliter of dilution. So, if you mix 1 ml of bacterial sample with 2 ml of saline solution you end with a total volume of 3 ml of diluted sample 1 ml / 3 ml = 0.33 (0.33 means that in your dilute sample every ml has 0.33 ml of the original bacteria culture) Another question that you may ask is: how many times has the sample being diluted? To figure this out we simply divide the initial concentration IC (which for the original sample is assigned the value of 1) by the final concentration FC. The resulting value is called the dilution factor DF. For our working example this would be: DF = IC/FC 1 / 0.33 = 3 the Dilution Factor (DF)for this dilution is 3 and it means that your original sample was diluted 3X You can use the dilution factor to relate back to the original sample. In the example above; the dilution factor indicates to us that in the original sample of bacteria there are 3 times as many bacteria/ml as in the diluted sample In some books you will find that they refer to The Dilution Factor as the number or factor used to multiply to obtain the original concentration of a sample. It is generally is calculated by the inverse or reciprocal of the dilution, DF = 1 / Dilution . In the example above DF = 1 / 0.33 = 3. THE SAME THING! But you may find that this way is easier for calculating DF. MICRB 202: Introductory Microbiology Lab Unit IV: Estimating Cell Density of Laboratory Cultures 7 Dilutions can be expressed as a exponent or as a ratio. An exponent is usually used only when working on dilutions dealing with factors of 10. When using ratios you should bear in mind that according to the American Society for Microbiology Style Manual , dilution ratios may be reported with either colons ( : ) or slash ( / ). It is very important that you be aware that there is a difference among them. For example, 1 / 2 and 1 : 2 ARE NOT THE SAME THING ! A slash ( / ) indicates that the ratio is part of a whole; so, 1 / 2 means 1 of 2 parts A colon ( : ) indicates the ratio is 1 part to 2 parts with a total of 3 parts So, How would you express the ratio 1 / 2 using a colon ( : ) ? Answer 1: 1 More Examples : a) If 1 ml of bacterial culture is mixed with 9 ml of water the dilution of the bacterial sample would be Dilution = 1ml/ 1+ 9 ml = 0.1 This dilution expressed as ratio would be 1:9 (for a total of 10 ml) or 1 / 10 Or, expressed as exponent 101 What is the dilution factor? DF = 1 / 0.1 = 10 b) If 0.5 ml of bacterial suspension is mixed with 9.5ml of water the dilution of the bacterial sample would be Dilution = 0.5 ml / 0.5 + 9.5 ml = 0.05, or 1 / 20, or 1:19 What is the dilution factor? DF = 1 / 0.05 = 20 c) 1 ml of bacterial suspension is mixed with 3 ml of diluent Dilution = 1 / 1+ 3 = 0.25, or 1 / 4 or 1: 3 DF = 1/ 0.25 = 4 Types of Dilution: In Biology and chemistry working with dilutions is a very important skills and there are several types of dilutions depending on what is to be accomplished. 1) Dilution of a solution to specified volume in one step : Usually it is used when there is a stock solution and from it a specific volume of the lesser concentration is required For example you have a stock solution of 30 % sugar and want to prepare 50 ml a solution containing 8% sugar. All you need is to calculate how much volume of the original stock solution you will need to mix with the diluent to end with 50 ml of 8% Use the formula C1 V1 = C2 V2 V1 = C2 x V2 / C1 = 8% x 50 ml / 30% = 13 ml you will need to take 13 ml of the 30% stock solution and mix it with the diluent to 50 ml 2) Dilution of a Solution to specified volume in several steps MICRB 202: Introductory Microbiology Lab Unit IV: Estimating Cell Density of Laboratory Cultures 8 This is needed for large dilutions. Sometimes the dilution have to be done in two or more steps. Example: You need to make 100 ml of a media containing 1 g/ml of the antibiotic ampicillin, you cannot weight 100 g but there is a stock solution of ampicillin containing 0.5 g /ml Still, this stock solution is 500,000 X (0.5 g x106 g/g = 500,000 /ml ) more concentrated than the required final concentration. This can be easily handled if the dilution is done in two steps. a) make a 10 ml of a stock with a concentration of 0.05 % and b) use a dilution from this 0.05% stock to make your media (in both cases you use the formula shown above. 3) Serial dilutions In serial dilutions a certain volume of material is being transferred from one vessel to another with the purpose of increasing the dilution of the material by certain increments. For example in a two fold serial dilutions the dilution factor is doubled each time Example: Make 3 two-fold serial dilutions from a bacterial culture. In addition, you are told to aim for a final volume for each dilution of 6 ml. This means that you start with the original culture 1/1 and from this, dilute down to produce 1 /2 , 1 /4, 1 /8 . You may use C1 V1 = C2 V2 i) to make the ½ from the stock culture C1 = 1/1 V1 = ? C2 = 1 /2 = 0.5 V2 = 6ml V1 = C2 x V2 / C1 0.5 x 6 ml / 1 = 3 ml ii) to make the ¼ from the ½ dilution C1 = 1/ 2 V1 = ? C2 = 1 / 4 = 0.25 V2 = 6ml V1 = C2 x V2 / C1 0.25 x 6 ml / 0.5 = 3 ml iii) to make the 1/8 from the ¼ dilution C1 = 1/ 4 = 0.25 V1 = ? C2 = 1 / 8 = 0.125 V2 = 6ml V1 = C2 x V2 / C1 0.125 x 6 ml / 0.25 = 3 ml