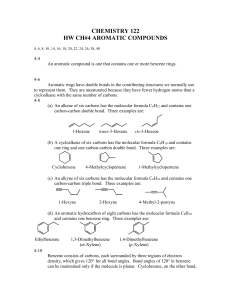
Identification of an Organic Acid
advertisement

Leah Hyde Chem 107 Lab Section A Identification of an Organic Acid Purpose: The purpose of this lab was to identify a general unknown acid. Important data such as the weight, solubility, and melting point were used to confirm the unknown. Tests were also preformed to identify whether the acid contained a benzene ring and if it contained a halogen. All of these properties were used in determining what the acid was and the molecular formation of the acid. Procedure: To determine the molecular weight of the unknown acid, a solution containing a given amount of the acid was titrated using a previously standardized sodium hydroxide solution (NaOH). To indicate when the solution was saturated, phenolphthalein was used as the indicator. Once the molecular weight was determined, several Lewis dot structures were drawn, utilizing the mass and considering isomers. The benzene ring and the halogen test were preformed along with finding the melting point to aid in the conformation of the unknown acid. After the molecular formula was obtained the data such as weight, solubility, melting point and molecular formula in accordance with the CRC Handbook was used to determine the correct form of the compound. 1 Leah Hyde Chem 107 Lab Section A Standardization of NaOH: I II III IV V VI VII VIII Mass of flask and sample (g) Mass of flask (g) g PAP Mole PAP 66.3565 90.6403 84.3178 89.6847 88.6691 99.0516 79.9501 91.9527 65.8503 90.1317 83.8128 89.1947 88.1821 98.5455 79.4334 91.4538 0.5062 .002479 .5086 .002491 .4798 .002349 .4900 .002399 .4870 .002385 .5061 .002479 .5167 .002531 .4989 .002443 Final buret reading (mL) Initial buret reading (mL) Base used (mL) Molarity of base (M) 33.55 31.35 26.08 22.50 42.70 25.20 18.30 34.30 17.10 16.50 11.89 6.10 27.50 9.37 2.07 18.50 16.45 14.85 14.19 16.40 15.20 15.83 16.23 15.80 .1671 .1677 .1655 .1463 .1569 .1617 .1559 .1546 *shaded columns represent the data used Sample calculation: 2 Leah Hyde Chem 107 Lab Section A Determination of the weight of a carboxylic acid: I II III G-477 G-477 G-477 Unknown number Mass of flask 90.1560 and sample (g) Mass of flask 89.6560 (g) 0.5000 Mass of sample (g) 89.7031 91.0012 89.2021 90.5032 .04992 0.4980 Final buret reading (mL) 30.51 29.51 29.90 Initial buret reading (mL) 8.61 7.65 8.15 Base used (mL) Moles of base used Weight (g) 21.90 21.86 21.76 .0034 .003405 .0033902 147.05 146.6 146.8 *shaded columns represent the data used Sample calculation: 3 Leah Hyde Chem 107 Lab Section A Melting point: Fast result ( wet range) 129.0-135.0 Slow result 134.5 Interpretation of molecular weight data, C-13 nmr, and experimental results: After completing the titrations, interpreting the C-13 nmr spectrum, finding the melting point, and performing the halogen and benzene ring tests, the molecular structure of the unknown acid was able to be determined. After three successful titrations of the unknown acid, the data showed that the acid weighed about 146.8 grams. The reason the acid is believed to weigh 146.8 grams is because the titrations produced three similar weights and when averaged together come out with a deviation equaling less then five parts per thousand, meaning that it is fairly accurate. After the weight was determined the molecular formula was narrowed down. Using the molecular weights of Carbon (C), Hydrogen (H), Fluorine (Fl), Chlorine (Cl), Bromine (Br), Iodine (I), Nitrogen (N), and Oxygen (O), the possibilities of the molecular formula are narrowed down. Since the unknown is an acid, it is to contain a carboxylic acid, or COOH group, within the molecular formula. So in determining the makeup of the compound the weight of the COOH group is subtracted from the initial weight of the unknown leaving the left over mass the new weight to work with. The COOH group weighs 45.02 grams and when subtracted from 146.8 it leaves about one hundred grams to work with. The next structure that is a possibility is the benzene ring. Not only can 4 Leah Hyde Chem 107 Lab Section A the data through numbers show that a ring is present in the molecular makeup of the molecule but there is also a benzene ring test that can be preformed to determine the presence of the ring. The benzene ring test requires the unknown to be placed on a spatula and if a very gray or black cloud of smoke appears after it has been burned in a flame, a benzene ring is present. A black cloud appeared so knowing that the benzene ring is present the weight of that, 77.1, is taken away from the hundred that was left. Being that there is about 26 grams to work with after the ring and the COOH group there are few possibilities left to try. Another indicator that can be used and that aids in the process of determining the weight is the C-13 nmr spectrum reading. The reading that went along with the unknown shows seven different carbons are in the molecule. The molecule may contain more than seven carbons, but no less. The spectrum picks up on different carbons. For example, if there are three sp3 carbons in a molecule but are all bonded to same elements or molecules and are identical, the nmr reading will only show one peak for those carbons. If they are not the same one peak will show up for each special carbon. Being that there are definitely six carbons in the ring and one from the carboxylic acid group that means there are seven carbons. But not all are different because the ring shows symmetry. There is 26 grams left; there is only room for two more carbons, because 2 more of anything else would make the molecular weight too much. So if on the ring there are two carbons coming off, each with two hydrogens attached, it leaves 7 different carbons, but the weight is still off. To figure out the remaining carbons and other elements that will fit all the information obtained, the nmr reading is used once again. The two remaining carbons must be double bonded with only one hydrogen coming off each because if they had two coming off each they would be 5 Leah Hyde Chem 107 Lab Section A sp3 and show a peak in the range of 0-50. There is no peak within that range so the unknown is that much closer to having an identity. The unknown now is known to contain the carboxylic acid group, a benzene ring, and a pair of double bonding carbons connecting the two structures to form C9H8O2. This formula appears to work because the molecular formula matches up with the previously obtained weight, but isomers still have to be sorted, along with the boiling point, which was found to be about 134.5 *C, to find the correct name for the unknown. To verify the correct name of the unknown acid based on the molecular formula and the boiling point, the CRC Handbook is used to sort through all isomers with the same molecular formula. There are 10 isomers which all are the molecular formula C9H8O2. All of their weights are the same, 148.16 g, solubility is similar, but they all have different boiling and melting points. An approximate melting point was obtained through, first finding a range where the unknown was wet, and second heating the unknown at a rate of about 2 degrees a minute to find it exact as possible. The melting point of the acid is about 134.5 degrees Celsius and out of the 10 isomers in the CRC Handbook, one matched it showing the closest melting point of 133.0. It also matched up with the solubility that the unknown had in ethanol. So the unknown acid matched up with the weight that was found out through titrations. Then the formula was found using that weight and the C-13 nmr spectrum reading and the benzene test. Finally the unknown was confirmed using the CRC Handbook and matching up with the boiling points and solubility. The unknown acid that was found in this experiment is 3-phenyl 2-propenoic acid (E). 6 Leah Hyde Chem 107 Lab Section A Name of Acid Mol. Formula Molecular Weight Melting Point/*C Solubility 2,5 dimethyl benzoic acid 3-phenyl 2propenoic acid (E) 3 phenyl 2propenoic acid (Z) C9H10O2 150.18 132 C9H8O2 148.16 133 H2O 1; EtOH 3; eth 3; lig2 H2O 1; EtOH 4; eth 3; ace 3 C9H8O2 148.16 42 EtOH 4; HOAc 4; lig 4 *shaded one is the unknown acid -The dimethyl benzoic acid was considered because the weight came close to the weight that was obtained through the titrations and the solubility and the melting point were close but eliminated after figuring out that it did not agree with the C-13 nmr results. -The e form of the 3 phenyl 2 propenoic acid was the correct acid because it matched up with the molecular weight, melting point, solubility, and agreed with the C-13 nmr results. -The z form of the 3 phenyl 2 propenoic acid was considered because the C-13 nmr results, weight and the solubility were accurate but immediately discarded when the melting point was too low. 7