Word Doc.
advertisement

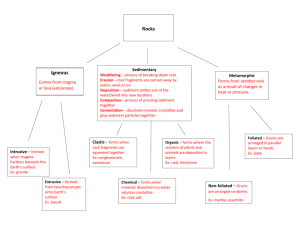
The Earth Rocks Exploration Phase Topic: Rock Types – Igneous, Sedimentary, and Metamorphic Activity I: Rock Identification Using Rock Kits 1. Using the rock kits provided determine which rocks samples are igneous, sedimentary and metamorphic. 2. Use the nail and magnifying lens to help you test the properties of each rock. 3. Place each rock you have identified on the appropriate corresponding sheet of paper labeled Igneous, Sedimentary or Metamorphic Rock. 4. Leave any rock samples you were not able to classify in the rock kit box. Activity II: Peer Review 1. Share your findings and review another group’s results from the initial rock identification. 2. Make suggestions as to which rocks may have been identified incorrectly and discuss the rock classifications amongst yourselves. 3. As we discuss the rock samples in class, note which rocks you identified correctly and which were incorrect. 2 Content Review: Igneous Rocks Igneous rocks – rocks that form when molten rock (rock liquefied by intense heat and pressure) cools to a solid state - when molten rock cools, it always forms a mass of intergrown crystals and/or glass; therefore, all igneous rocks have a crystalline or glassy texture Igneous Processes Intrusive vs. Extrusive Igneous Rocks Intrusive – rocks that form by the cooling of intrusions within the earth Extrusive – igneous rocks that form at the planet’s surface; they are extruded or violently ejected onto the surface Bodies of Igneous Rocks Batholith – massive igneous intrusions (covering regions of 100 km2) Sill – sheet-like intrusions that force their way between layers of rock Laccolith – blister-like sills Pipe – vertical tubes that feed volcanoes Dike – sheet-like intrusions that cut across layers of rock (sheet dike, ring dike, radial dike) Textures - dependent on nucleation; the atoms are mobile in a magma and free to nucleate nucleation - initial formation of a microscopic crystals to which other atoms progressively bond - generally if magma cools more slowly, the atoms in the magma have time to grow - if the magma cooled quickly, the mineral crystals will be smaller because they didn’t have time to grown and nucleate obsidian - formed when the intense heat of a volcano fuses masses of silica together very quickly forming the hard glass 3 Examples of Igneous Rock Textures Pegmatitic – crystals > 1cm, very slow cooling Phaneritic – crystals 1-10mm, slow cooling Porphyritic – large and small crystals (phenocrysts = large crystals, matrix = smaller more numerous minerals Aphanitic – crystals < 1 mm, rapid cooling, fluid lava Glassy – rapid cooling Vesicular – rapid cooling of gas charged lava, gas bubbles in lava (scoria, pumice) Pyroclastic – particles or fragments emitted from volcanoes (tuff – volcanic ash, volcanic breccia) Mineral Composition Mafic vs. Felsic Minerals Mafic minerals (dark-colored) – ferromagnesian minerals (iron and magnesium) Biotite Mica (black) Amphibole (dark gray) Pyroxene (dark green) Olivine (green) Felsic minerals (light-colored) Quartz (gray) Plagioclase feldspar (white) Potassium Feldspar (K-spar) (pink) Muscovite mica (brown) Color Index (CI) – is the percentage (by volume) of mafic mineral crystals in the rock - a rough measure of the proportions of mafic and felsic minerals Felsic igneous rocks: 0-15% mafic minerals Intermediate igneous rocks: 16 – 45% mafic minerals Mafic igneous rocks: 46-85% mafic minerals Ultramafic igneous rocks: 86-100% mafic minerals 4 Sedimentary Rocks How do sedimentary rocks form? They form when sediments accumulate and are lithified (meaning hardened by compression or cementation), or when masses of intergrown mineral crystals precipitate from aqueous solutions - Lithification of sediments - Cementation – thin films of chemical residues “glue” grains together - Precipitation of aqueous solutions (i.e. rock salt) Sediments – these are loose grains and chemical residues of rock fragments, mineral grains, animals or plants, and rust (hydrated iron oxide residue) - Sediments are the products of weathering processes - All sediments have a source; either produced by biochemical (organic) processes of plants and animals or by chemical or physical weathering processes of organic and inorganic materials Physical weathering – the cracking, scratching, crushing, abrasion, or other physical disintegration - Large rocks will be broken down into individual clasts (broken pieces of rock fragments and minerals) - Plant matter, logs and animal shells will be broken down into peat and shell gravel Chemical weathering - the chemical decomposition or dissolution of earth materials - Feldspar and mica -- broken down into clay minerals - Calcite -- goes to calcium and bicarbonate ions in solution 5 Classification of Sedimentary Rocks A) Clastic (a.k.a. Detrital) – rocks made up of mostly rock fragments, quartz grains, feldspar grains, or clay minerals B) Biochemical (a.k.a. Bioclastic) – rocks made mostly of grains that are fragments or shells of organisms (plant/animals) C) Chemical – made mostly of mineral crystals precipitated from aqueous solutions and/or chemical residues (e.g. rust) Texture of a Sedimentary Rock - Texture is a description of its constituent parts and their sizes, shapes and arrangements - Sediments can be transported great distances by wind, water and ice - This causes the sediment grains to be dragged, bounced, rolled, and carried and causes grains to be scratched, broken, rounded and worn (glacial action) Grain Shape Angular grains Subangular Subrounded Rounded Grain Sizes Gravel - includes grains larger than 2 mm in diameter (granules, pebbles, cobbles, boulders) Sand - all grains are visible and feel gritty between your fingers (1/16mm-2mm in diameter) Silt - grains usually too small to see (grains from 1/256mm to 1/16mm in diameter) 6 - You can feel them as tiny grits between your fingers or teeth Clay - grains too small to see w/out microscope (grains less than 1/256mm in diameter) - Feels smooth when rubbed between your fingers Sorting of Sediments (Grain Arrangements) – wind and water currents transport and naturally separate sediments into different grains sizes and densities Well– sorted – composed of sediments that are of similar sizes; usually well-rounded Poorly– sorted – many different sizes and/or densities of sediment grains mixed together Metamorphic Rocks Metamorphic Rocks – rocks that are changed from one form to another by intense heat and pressure or by the action of watery hot fluids inside of the earth - Examples: marble, quartzite, slate, phyllite, schist, gneiss, metaconglomerate, anthracitic coal, etc. Parent Rock – the original rock that was metamorphosed into a new rock; also known as protolith; can be any of the three types of rocks (igneous, sedimentary or metamorphic) - Examples: limestone, sandstone, shale, conglomerate, mafic or ultramafic igneous rx Common Metamorphic Rock forming Minerals: Quartz, Calcite, Dolomite, Feldspars, Muscovite, Biotite, Chlorite, Garnet, Tourmaline, Serpentine, Talc, Kyanite, Sillimanite, Amphibole (Hornblende) Contact metamorphism – occurs locally, adjacent to igneous intrusions and along fractures that are in contact with watery hot fluids (hydrothermal metamorphism) 7 - Hydrothermal Metamorphism – involves the condensation of gases to form liquids which can precipitate mineral crystals along fractures - Zones of contact metamorphism are narrow from millimeters to tens of meters thick - Caused by conditions of moderate pressure and heating for days to thousands of years Regional metamorphism – occurs over very large areas (regions), such as deep within the cores of rising mountain ranges, and is generally accompanied by folding and shearing of rock layers - Caused by 1) large igneous intrusions that form and cool over long periods, 2) extreme pressure and heat, and/or 3) widespread migration of hot fluids * Note: most major intrusions are preceded by contact metamorphism and then followed by regional metamorphism Recrystallization – a process where small crystals of one mineral will slowly convert to fewer, larger crystals of the same mineral, without melting the rock Metamorphic Rock Textures 1) Foliated: rocks with a layered appearance Slaty rock cleavage – very flat foliation developed along closely spaced shear planes (slate) Phyllite texture – wavy to wrinkled foliation of cryptocrystalline platy minerals (phyllite) Schistosity – a scaly, glittery layering of visible platy minerals (medium-tocoarse-grained), and/or linear alignment of long prismatic crystals (schist, garnet schist) 8 Gneissic banding – alternating layers of light and dark medium-to-coarse-grained minerals (gneiss) 2) Non-Foliated: no obvious layering present Crystalline texture – aggregate of intergrown crystals, medium-to-coarse grained (marble) Microcrystalline texture – very fined-grained aggregate of intergrown crystals (hornfels) Sandy texture – medium-to-coarse-grained aggregate of fused sand-sized grains (quartzite) Glassy texture – no visible grains (anthracite coal) 3) Other Metamorphic Features Stretched or sheared grains – deformed pebbles, fossils, or mineral crystals that have been stretched out (i.e. metaconglomerate) Porphyroblastic texture – large crystals set in a finer-grained groundmass Hydrothermal veins – fractures healed by minerals that precipitated from hydrothermal fluids Folds – bends and buckles in rock layers that were originally flat Lineations – lines on rock at the edges of foliation, shear planes, etc. 9 Application Phase Activity: 1. Pass around and discuss the rock hand samples (larger classroom samples) and determine what minerals comprise each rock. 2. Classify the hand samples by type (igneous, sedimentary or metamorphic). Terms: Parent rock, igneous, metamorphic, sedimentary, batholiths, intrusion, mafic, felsic, pyroclastics, lithifaction, foliation, color index, sedimentation, weathering, metamorphosis, extrusion, layering, pressure, heat, texture. 10 Teacher Notes: Additional Classroom Learning Activities Magma Lab Activity Objectives When you have completed this lab you should be able to: 1. explain why magma rises through the lithosphere, often making it to the surface and out of a volcano. 2. describe the process of crystallization and how the rate of cooling of a melt affects the sizes of the crystals formed. Activity #1: Why Does Magma Rise? Materials: covered test tube of salol (phenyl salicylate) plastic beaker of hot tap water (get from front sink) hot plate large glass beaker with a little boiling water in it thermometer insulated gloves test tube rack empty test tube crushed ice Activity 1. Melt almost all of the salol: Measure the temperature of the hot tap water. If it is below 120°, pour some out and add a little boiling water. Hold the test tube of salol in the hot water, swirling it around gently. Periodically remove the test tube from the hot water and see if the salol has melted. Continue this process until the crystals are almost all melted (leave a piece of crystalline salol, about 1/8 inch across, in the melt to act as a seed crystal). This process is analogous to the melting of rock deep within Earth’s crust or mantle. 2. Place the test tube of salol in an upright position in the metal test tube rack. 3. Fill the unsealed empty test tube about 1/3 full of tap water. Place a few pieces of crushed ice into the water (if the ice melts, just add a little more ice). Questions: 1. Draw two diagrams, one showing the seed crystal inside the test tube of melted salol and one showing the crushed ice inside the test tube of water. Test tube with a few crystals of salol in molten salol Test tube with a few pieces of crushed ice in water 11 2. Which has a higher density, crystalline (solid) salol or molten (liquid) salol? How do you know? 3. Which has a higher density, water or ice? How do you know? 4. When rock melts, deep under ground, it typically isn't any hotter than the unmelted rocks around it; it merely has a lower melting temperature than the rocks around it. Yet, the melt (magma) tends to rise, often making it all the way to the surface as a lava flow. Why does magma begin to rise, even though it's no hotter than the unmelted rocks around it? Activity #2: Melting and Crystallization Materials: 2 Petri dishes, containing salol (phenyl salicylate) hot plate insulated gloves 1 metal bowl with ice on the bottom (get ice from front lab table) 10x magnification hand lenses large example of radiating clumps of crystals (in a box) large example of a single crystal (in a box) Make a Prediction: In this activity, you will be melting and then cooling (and therefore crystallizing) molten salol at two different speeds. The possible results are as follows: a. The salol whose temperature drops faster will form larger crystals. b. The salol whose temperature drops more slowly will form larger crystals. c. The rate of cooling will not make any difference; the crystals will be the same size, no matter how quickly the temperature of the salol drops. Choose the result that you think will occur. Explain the reasoning behind your answer. 12 Activity 1. Melt the salol: Set the hot plate on low. CAREFULLY—supporting the bottom of the Petri dish so that it doesn't fall, place the Petri dish on the hot plate with one side hanging 1/4 inch or so over the edge. Let all the salol melt except for a small amount at the overhanging edge. 2. Remove the salol from the hot plate: Wearing the insulated gloves, CAREFULLY— supporting the bottom of the Petri dish so that it doesn't fall—remove each Petri dish from the hot plate and place it on the lab table. 2. Simulate the formation of a volcanic rock: Place one of the Petri dishes on hte bowl of ice, causing it to cool rapidly. This rapid cooling process is analogous to the formation of a volcanic rock; the melted rock (lava) cools and crystallizes quickly because it erupts onto the Earth's surface, which is much cooler than the depths of the Earth. Look at the crystals with a hand lens; note the sizes of the crystals. 3. Simulate the formation of a plutonic rock: Meanwhile, back at the lab table, the remaining Petri dish has been cooling slowly. This slow cooling process is analogous to the formation of a plutonic rock; the melt cools and crystallizes slowly because it stays deep underground and has a thick insulating layer of rock above it. Look at the crystals with a hand lens; note the sizes of the crystals. Questions: 1. Which procedure produces larger crystals, a rapid temperature drop or a gradual temperature drop? Why? Hint: Be sure to base your answer on the sizes of individual crystals; not on clumps of small radiating fibrous crystals (see the large example of similar clumps of crystals). Large individual crystals of salol are diamond shaped if they are free to grow without bumping into other crystals (see the large example of a similar crystal). 2. Draw enlarged sketches of some of the crystals in each test tube. 13 Crystals that formed when the salol cooled quickly Crystals that formed when the salol cooled slowly 3. Which should have larger crystals, volcanic rock or plutonic rock? Explain the reasoning behind your answer. 4. What would happen if the melt were chilled so suddenly that the crystals had no time to form? Why? 5. In terms of crystal size, what would happen if the liquid salol cooled slowly for awhile and then was cooled quickly (placed in ice water)? Explain the reasoning behind your answer. If there's time, try it! 5. If magma cools slowly deep underground for awhile and is then expelled quickly onto the surface, will the crystals be big or small? Explain the reasoning behind your answer. 14 Activity #3: Watching the Crystallization Process Materials: glass Petri dish full of salol (phenyl salicylate), with glass cover. hot plate 10x magnification hand lenses insulated gloves paper towels Activity 1. Melt the salol: Set the hot plate on low. CAREFULLY, supporting the bottom of the Petri dish so that it doesn't fall, place the Petri dish on the hot plate with one side hanging 1/4 inch or so over the edge. Let all of the salol melt except for a small amount at the overhanging edge. 2. Remove the salol from the hot plate: Wearing the insulated gloves, CAREFULLY— supporting the bottom of the Petri dish so that it doesn't fall—remove the Petri dish from the hot plate and place it on the lab table. If the cover glass fogs up (usually it does), briefly place the cover glass upside down on the hot plate; then wipe the inside with a paper towel and put it back on the Petri dish. 3. Watch the salol crystallize again: Using the magnifying hand lens, watch the crystals form and grow. Questions: 1. Do crystals start growing all over the dish or do they start in a few spots and grow bigger from there? Describe what happened. 2. Try repeating the experiment but place the dish on a bed of ice. This time, do the crystals start growing all over the dish or do they start from a few spots and grow bigger from there? 15 Sedimentary Processes Lab Objectives When you have completed this lab activity, you should be able to: 1. explain the essential difference between chemical sediment and detrital sediment. 2. describe how and why detrital sediment is deposited. 3. describe how and why chemical sediment are deposited. 4. explain how the speed of flowing water affects (a) the sizes of detrital sediment particles that the water can carry and (2) the sizes of detrital sediment particles that the water deposits. 5. explain why detrital sediment is often layered by particle size. 6. distinguish two very different mechanisms by which crystals can grow in a fluid. 7. explain how sediment is transported from far inland to the sea. 8. describe how running water can transform a featureless terrain into a complex landscape of ridges and valleys. 9. distinguish between erosion and deposition. 10. identify the following features of a river: tributaries, trunk stream, delta, distributaries Important Definitions Dissolved: a substance is dissolved in a fluid (liquid or gas) when its component ions, atoms or molecules have become separated and individually surrounded by molecules of the fluid. Sediment: solid material that has settled to the ground or to the bottom of a body of water. Chemical Sediment: Sediment that was once dissolved in water. Detrital Sediment: Sediment that was never dissolved in water. 16 Activity #1: Chemical vs. Detrital Sediment Materials: 1 clear plastic cup containing fine-grained halite (table salt) 1 clear plastic cup containing powdered clay water 2 stirring rods Prediction: Which do you think will dissolve in water: the salt, the clay, neither or both? Explain the reasoning behind your answer. Activity: • Add water to each cup until it is approximately 2/3 full. • Thoroughly stir the contents of each cup for at least a minute. • Observe each cup right after you have finished stirring. Questions: 1. Draw diagrams of the two cups immediately after you have finished stirring. Water + Salt Water + Clay 2. Which substance dissolved in the water: the clay, the salt, neither or both? Use your observations of the two cups to justify your answer. More Activity: • Let the two cups rest undisturbed on the lab table for an hour or so. More Questions: 3. Draw diagrams of the two cups after they have rested on the lab table for an hour or so. 17 Water + Salt Water + Clay 4. Explain why the distribution of sediment in the two cups is so different. 5. Clay is a Salt is a detrital detrital / / chemical chemical sediment (circle the correct answer). sediment (circle the correct answer). Activity #2: Causing the Precipitation of Chemical Sediment from a Solution Materials: salt water (solution of sodium chloride in water) spoon one glass Petri dish 10x magnification hand lenses metal stand with a heat lamp on it, pointing down Activity 1. Place a few spoonfuls of salt water into the Petri dish. Look at the solution with a hand lens. Draw a diagram of the Petri dish in the space provided below (Question 1). 2. Place the Petri dish under the heat lamp and let the water gradually evaporate. Go on and do Activities 3 and 4. After 10-15 minutes, examine the Petri dish and answer the questions below. Questions: 1. Draw diagrams of the Petri dish, before and after the water evaporated. 18 Before Water Evaporated After Water Evaporated 2. What caused the crystals to form in the Petri dish? 3. How is the process that formed these crystals fundamentally different from the process that formed the crystals of salol in the test tubes (you did this a couple of weeks ago)? 19 Activity #3: Studying the Deposition of Detrital Sediment Materials: Mason jar with water and detrital sediment in it. Dry detrital sediment: Mixture of gray pea gravel, fine white sand and a small amount of dark silt (the black "dirt" in central and western Chico is ideal). Activity: 1. Shake the jar vigorously; the water picks up the detrital sediment, just as a flowing stream does. 2. Stop shaking the jar and watch what happens to the detrital sediment as the water slows down and comes to rest. Questions: 1. What causes the detrital sediment to become suspended in the water? 2. What causes the detrital sediment to settle to the bottom of the jar? 3. Do the smallest grains land on the top or on the bottom of the layer? Why? 4. What is the important factor in determining which grains land on the bottom of the layer, their weight or their density? Why? (Hint: what happens to the density of a large boulder when it's broken into small pieces?) 5. What natural river or ocean processes could result in the deposition of detrital sediment? 20 6. After most of the detrital sediment has settled, draw a diagram of the sediment in the jar, noting especially any variations in the size and/or color of the sedimentary particles. More Activity: Open the top of the jar and add another 1/4 cup or so of sediment; watch it settle. Repeat several times. Note the layering; such layering is always present in sedimentary rock. Questions: 7. Draw a diagram of the multiple layers of detrital sediment in the jar, noting especially any variations in size and/or color of the sediment. 8. Describe a natural scenario that could result in the deposition of distinct layers of detrital sediment. 21 Activity #4: Watching Running Water Modify a Landscape1 Materials: 2 large plastic trays, each with a small hole in one end small rubber stopper moistened sediment (diatomaceous earth--the kind used for swimming pool filtration- mixed with a small amount of fine sand) flat plastic spatula large (1 or 2 liter) plastic beaker spray bottle full of water sponge Activity: 1. Initial set-up a. The sediment in your tray should be pre-moistened. If it is not, ask your instructor to do it. b. Plug the hole in the tray with the rubber stopper. Mix the moistened sediment well, using the plastic spatula and/or your hands. You will have to scrape the sediment off of the bottom using lots of elbow grease. Mix the sediment well until the sand (tan) is evenly distributed in the diatomaceous earth (white). If necessary, add some water until the sediment has the consistency of a mud pie. c. Turn the empty tray upside down. Rest the tray containing the moistened sediment on the edge of the empty tray, with the plugged hole on the uphill side of the tray. Rubber stopper Empty Tray Tra y Co ntai ning M oistene d Se dim e nt d. Push the sediment to the side of the tray opposite the hole. The sediment should cover about half of the bottom of the tray. e. Firmly pat the sediment with your hands to make its upper surface as flat as possible (it will become very soupy when you do this). f. Use the sponge to clean as much sediment as possible from the exposed bottom of the tray. 1This laboratory activity was modified from a) The River Cutters unit of the Great Explorations in Math and Science (GEMS) curriculum materials for Grades 6–9, published in 1989 by the Lawrence Hall of Science, University of California at Berkeley. b) The Stream Tables activity of the Landforms Module of the Full Option Science System (FOSS) curriculum materials for grades 5–6, published in 1990 by the Lawrence Hall of Science, University of California at Berkeley; distributed by Encyclopaedia Brittanica. 22 Comments: This is a small-scale model of a landscape. An inch on the model represents about a mile on a real landscape. A grain of sand represents a boulder. Regarding evolution of the landscape, one minute of the experiment is equivalent to about 1000 years in real life. 2. Running the Experiment: a. Gently and slowly (so as not to disturb the sediment), place the tray of sediment flat on the upside-down tray. b. Fill the plastic beaker with water and SLOWLY pour water into the sediment-free side of the tray just until there are no more dry spots on the bottom of the tray. This water represents the ocean; the sediment represents the land. Shoreline Land Ocean c. Watch as water drains off of the “land.” It will form several streams. At first, nothing may appear to be happening. Be patient and keep watching. Try to be the first in your group to see a stream appear. d. Continue watching as the running water carves a landscape by eroding, transporting and depositing sediment. Be sure to watch what happens in the ocean as well as what happens on land. Questions: 1. Where, in the model, is erosion occurring? 2. Where in the model is deposition occurring? 3. Explain the essential difference between erosion and deposition. 4. What is the predominant sediment that is eroded from the land, transported by the streams, and deposited into the ocean? The diatomaceous earth (white) or the sand (tan)? Why? 23 5. Is sediment deposited as one even layer in the ocean, or is more sediment deposited near the shoreline? Explain why this occurs. More Activity: Continue Running the Experiment: a. Gently spray a fine mist of water over the land (a few “squirts” should be enough)--you have just made it rain! b. Watch the water run off the land. Notice how efficiently the streams channel the water from the land to the ocean. c. Repeat several times, causing the landscape to continue to evolve. 24