Chemistry II Solubility Curves Name
advertisement

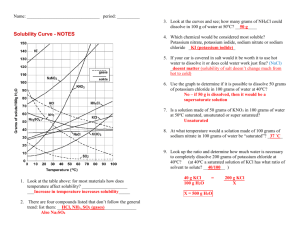
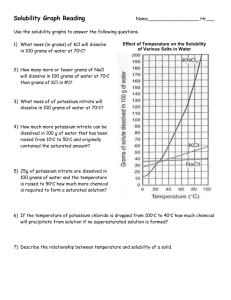
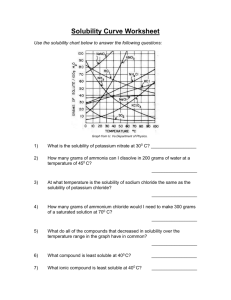
Chemistry II Solubility Curves Name: __________________ DIRECTIONS: Use the handout graph to solve the following problems. 1. What is the solubility of potassium nitrate in 100 grams of water at 60oC? 2. What is the solubility of potassium chloride in 100 grams of water at 60oC? 3. What is the solubility of sodium chloride in 100 grams of water at 90oC? 4. What is the minimum temperature needed to dissolve 65 grams of potassium nitrate in 100 grams of water? 5. What is the minimum temperature needed to dissolve 35 grams of potassium chloride in 100 grams of water? 6. At what temperature do potassium chloride and potassium nitrate have the same solubility? 7. At what temperature do potassium nitrate and sodium chloride have the same solubility? 8. If 20 grams of potassium chlorate are mixed with 100 grams of water at 40oC, how much will not dissolve? 9. If 200 grams of potassium nitrate are mixed with 100 grams of water at 70oC, how much will not dissolve? 10. If 50 grams of sodium chloride are mixed with 100 grams of water at 90oC, how much will not dissolve? 11. If 75 grams of sodium nitrate are added to 100 grams of water at 35oC, how much more must be added to saturate the solution? 12. If 120 grams of potassium iodide are added to 100 grams of water at 15oC, how much more must be added to saturate the solution? 13. If 10 grams of sodium chloride are added to 100 grams of water at 100oC, how much more must be added to saturate the solution? 14. 100 grams of water are saturated with sodium nitrate at 30oC. If this solution is heated to 60oC, how much more can be dissolved? 15. 100 grams of water are saturated with potassium nitrate at 0oC. If the solution is heated to 65oC, how much more can be dissolved? 16. Which two substances have the same solubility at 75oC? 17. 100 grams of water at 70oC are saturated with potassium chlorate. If this solution is cooled to 25oC, how much of the solid will precipitate (change, from the dissolved state to the solid state)? 18. At what temperature do sodium nitrate and potassium nitrate have the same solubility? 19. How much potassium nitrate will dissolve in 50 grams of water at 50oC? 20. How much potassium nitrate will dissolve in 10 grams of water at 50oC? Chemistry II Solubility Curves Name: _______Key_ DIRECTIONS: Use the handout graph to solve the following problems. 1. What is the solubility of potassium nitrate in 100 grams of water at 60oC? 105g KNO3 2. What is the solubility of potassium chloride in 100 grams of water at 60oC? 45 g KCl 3. What is the solubility of sodium chloride in 100 grams of water at 90oC? 4o g NaCl 4. What is the minimum temperature needed to dissolve 65 grams of potassium nitrate in 100 grams of water? 38 5. What is the minimum temperature needed to dissolve 35 grams of potassium chloride in 100 grams of water? 35 6. At what temperature do potassium chloride and potassium nitrate have the same solubility? 20 7. At what temperature do potassium nitrate and sodium chloride have the same solubility? 25 8. If 20 grams of potassium chlorate are mixed with 100 grams of water at 40oC, how much will not dissolve? 5g 9. If 200 grams of potassium nitrate are mixed with 100 grams of water at 70oC, how much will not dissolve? 65g 10. If 50 grams of sodium chloride are mixed with 100 grams of water at 90oC, how much will not dissolve? 10g 11. If 75 grams of sodium nitrate are added to 100 grams of water at 35oC, how much more must be added to saturate the solution? 25g 12. If 120 grams of potassium iodide are added to 100 grams of water at 15oC, how much more must be added to saturate the solution? 20g 13. If 10 grams of sodium chloride are added to 100 grams of water at 100oC, how much more must be added to saturate the solution? 30g 14. 100 grams of water are saturated with sodium nitrate at 30oC. If this solution is heated to 60oC, how much more can be dissolved? 30g 15. 100 grams of water are saturated with potassium nitrate at 0oC. If the solution is heated to 65oC, how much more can be dissolved?115g 16. Which two substances have the same solubility at 75oC?NaCl and KClO3 17. 100 grams of water at 70oC are saturated with potassium chlorate. If this solution is cooled to 25oC, how much of the solid will precipitate (change, from the dissolved state to the solid state)? 25g 18. At what temperature do sodium nitrate and potassium nitrate have the same solubility? 68 19. How much potassium nitrate will dissolve in 50 grams of water at 50oC? 42.5g 20. How much potassium nitrate will dissolve in 10 grams of water at 50oC? 8.4g