Kinetics - Bakersfield College
advertisement
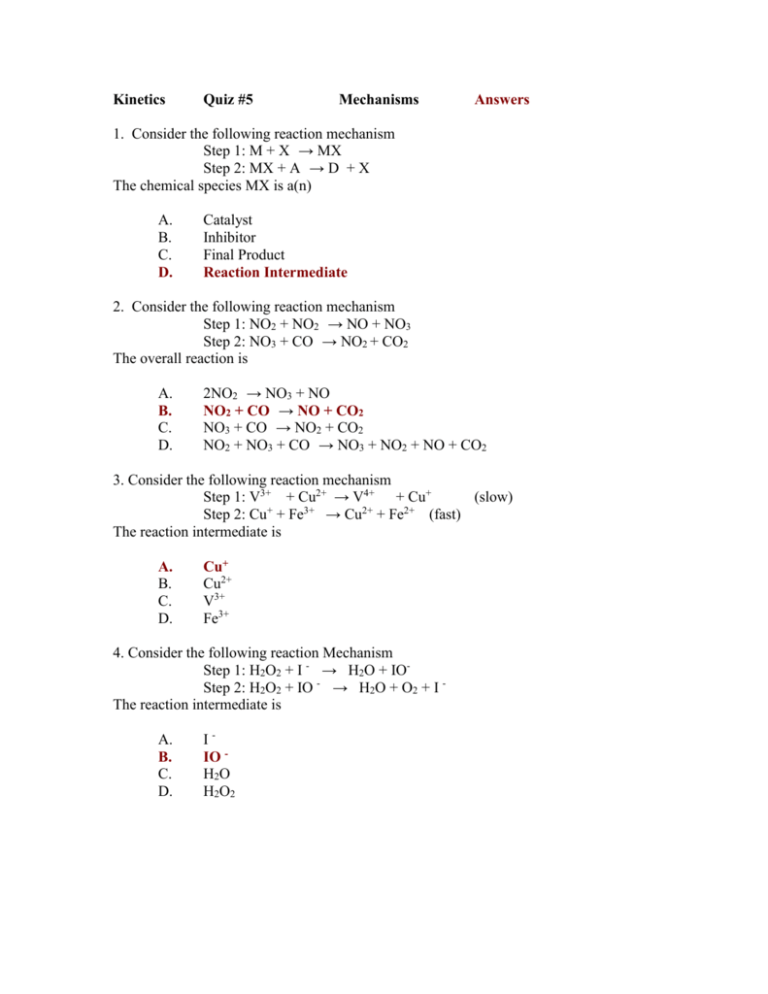
Kinetics Quiz #5 Mechanisms Answers 1. Consider the following reaction mechanism Step 1: M + X → MX Step 2: MX + A → D + X The chemical species MX is a(n) A. B. C. D. Catalyst Inhibitor Final Product Reaction Intermediate 2. Consider the following reaction mechanism Step 1: NO2 + NO2 → NO + NO3 Step 2: NO3 + CO → NO2 + CO2 The overall reaction is A. B. C. D. 2NO2 → NO3 + NO NO2 + CO → NO + CO2 NO3 + CO → NO2 + CO2 NO2 + NO3 + CO → NO3 + NO2 + NO + CO2 3. Consider the following reaction mechanism Step 1: V3+ + Cu2+ → V4+ + Cu+ (slow) Step 2: Cu+ + Fe3+ → Cu2+ + Fe2+ (fast) The reaction intermediate is A. B. C. D. Cu+ Cu2+ V3+ Fe3+ 4. Consider the following reaction Mechanism Step 1: H2O2 + I - → H2O + IOStep 2: H2O2 + IO - → H2O + O2 + I The reaction intermediate is A. B. C. D. IIO H2O H2O2 5. Consider the following potential energy diagram PE (KJ) Progress of the reaction The above potential energy diagram represents an A. B. C. D. Exothermic reaction involving one step Exothermic reaction involving two steps Endothermic reaction involving one step Endothermic reaction involving two steps 6. Consider the following reaction mechanism Step 1: NO2(g) + NO2(g) → NO(g) + NO3(g) (slow) Step 2: NO3(g) + CO(g) → CO2(g) + NO2(g) (fast) Which one of the following changes would result in the greatest increase in reaction rate A. B. C. D. Increase [CO] Decrease [NO] Increase [NO2] Increase [NO3] 7. An uncatalyzed reaction was found to produce 40 kJ of energy in 10 mins. When catalyzed, the same reaction produced 40 kJ of energy in 2 mins. Which one of the following potential energy diagrams is consistent with the above data? PE A B PE Reaction Path C PE Reaction Path D Reaction Path PE Reaction Path 8. Consider the following reaction mechanism Step 1: ICl + H2 → HI + HCl (slow) Step 2: ICl + HI → HCl + I2 (fast) The Species HCl is a A. B. C. D. Product Catalyst Reactant Reaction Intermediate 9. Consider the following reaction mechanism Step 1: Cl(g) + O3(g) → ClO(g) + O2(g) Step 2: O(g) + ClO(g) → Cl(g) + O2(g) The Reaction intermediate is A. B. C. D. Cl O2 O3 ClO 10. In a reaction mechanism, the rate determining step is the A. B. C. D. Fastest and has the lowest reaction rate. Fastest and has the highest activation energy Slowest and has the lowest activation energy Slowest and has the highest activation energy PE Reaction Progress 11. Select the true statement concerning the above potential energy diagram. The catalyzed reaction has a larger ∆H The uncatalyzed reaction has a larger ∆H The catalyzed reaction has a greater rate of reaction The uncatalyzed reaction has a greater rate of reaction A. B. C. D. 12. Which point on the diagram above represents the potential energy of the activated complex formed in the uncatalyzed reaction? I II A. B. C. D. III IV I II III IV 13. Consider the following reaction Step 1: NO(g) + O3(g) ------ > NO2(g) + O2(g) Step 2: O(g) + NO2(g) ------ > NO(g) + O2(g) The catalyst is A. B. C. D. O2 O3 NO NO2 14. Consider the following reaction mechanism Step 1: N2O(g) ------ > N2(g) + O(g) Step 2: N2O(g) + O(g) ------ > N2(g) + O2(g) The reactant in the overall reaction is A. O B. C. D. O2 N2 N2O 15. Consider the following reaction O3(g) + NO(g) ----- > NO2(g) + O2(g) NO2(g) + O(g) ------ > NO(g) + O2(g) The product in the overall reaction is A. B. C. D. O2 O3 NO NO2 Use the following reaction mechanism to answer questions 16 and 17. Step 1: 2PO → P2O2 (fast) Step 2: P2O2 + H2 → P2O + H2O (slow) Step 3: P2O + H2 → P2 + H2O (fast) 16. Increasing the concentration of which of the following substances would cause the greatest increase in the reaction rate? A. H2 B. PO C. P2O D. H2O 17. Which of the following are products in the overall reaction? I P2 II P2O2 III PO2 IV H2O A. I and II only B. I and IV only C. II and III only D. III and IV only 18. The Intermediates are: A. NO H2 B. H2O P2 C. P2O2 PO D. P2O2 P2O 19. Which of the following could describe a catalyst? A. A substance that increases the reaction time. B. A substance that provides an alternate mechanism with higher activation energy. C. A substance that is formed in one step and used up in a subsequent step in a reaction mechanism. D. A substance that is used up in one step and reformed in a subsequent step in a reaction mechanism. 20. Consider the following reaction: 2PO(g) + O2(g) → 2PO2(g) Why would this reaction probably involve more than one step? A. There is insufficient activation energy. B. This reaction has high activation energy. C. Reactions between gases are typically slow. D. A successful collision between three molecules is unlikely. 21. Consider the following reaction mechanism: Step 1 Cl3 → Cl2 + Cl Step 2 Cl3 + Cl → 2Cl2 Which of the following could represent the activated complex for Step 2? A. Cl B. Cl2 C. Cl3 D. Cl4 Consider the reaction: 2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(l) Which of the following explains, in terms of collision theory, why this reaction occurs in more than one step? A. a low C4H12(g) pressure B. low temperature of reactant mixture C. low probability of a multi-particle collision D. particles collide with insufficient kinetic energy 22. 23. Consider the following reaction mechanism and overall reaction: Step 1 ½ O2(g) + NO(g) → NO2(g) sunlight Step 2 Step 3 O2(g) NO2(g) + O(g) → → O3(g) Overall 3/2 O2 (g) → What is the catalyst in this mechanism? A. O B. NO C. NO2 D. sunlight NO(g) O3(g) + O(g) 24. What is an intermediate in this mechanism? A. O B. NO C. O3 D. sunlight 25. Consider the following reaction mechanism: Step 1 NOI → NO Step 2 NOI + I → NOI2 Identify the reaction intermediate. A. I B. NOI2 C. NO D. NOI 26. Consider the following reaction mechanism: Step 1 2NO2 → NO3 + NO Step 2 NO3 + CO → NO2 + CO2 Identify a product in the overall reaction. A. CO B. CO2 C. NO2 D. NO3 27. Consider the following reaction mechanism: Step 1 2NO2 → NO3 + NO Step 2 NO3 + CO → NO2 + CO2 Identify an intermediate. A. CO B. CO2 C. NO2 D. NO3 + I






