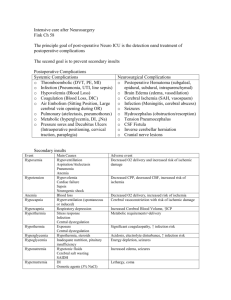
neurocritical care for the house officer
advertisement

NEUROCRITICAL CARE FOR THE HOUSE OFFICER1 Christine A.C. Wijman, MD, PhD Marion Buckwalter, MD, PhD Anna Finley Caulfield, MD Chitra Venkatasubramanian, MBBS, MD Division of Neurocritical Care Stanford Stroke Center INTRODUCTION Welcome to the Neurocritical care rotation. Advances in the diagnosis and treatment of neurological disease have recently led to a dramatic expansion in the field of Neurocritical care. In 2003 the Neurocritical Care Society was founded in Phoenix, AZ, and the first issue of the journal Neurocritical Care appeared in January 2004. As most patients with life-threatening neurological diseases have systemic disease, the critical care unit facilitates an interdisciplinary approach to patient care that involves neurology, neurosurgery, trauma, anesthesiology and pulmonary/critical care medicine, among others. Patients should receive neurocritical care if they have signs of raised intracranial pressure, coma, or neurological disease associated with respiratory or cardiovascular failure. Other patients who benefit from neurocritical care include those with subarachnoid hemorrhage, stroke, meningitis, encephalitis, status epilepticus, progressive muscular weakness and head trauma. Patients who undergo neuro-interventional radiological procedures may also benefit from neurocritical care. The Neurocritical care team consists of an attending, fellow, 1-2 residents, and 0-1 medical students. The Neurocritical care or Stroke fellow is expected to coordinate care with the residents and students on service, as well as the residents and fellows on the medical ICU and neurosurgery teams. In practical terms, this means that prior to morning rounds, the fellow will preround on any patients that are over the 8 per resident Neuro-ICU cap, see any patients with acute issues who the NICU resident has questions about, and help the resident and student formulate a plan of care on their patients. NICU residents will be assigned their patients by the fellow, and while the residents will tend to primarily cover neurology rather than neurosurgery patients, patients should be assigned to the residents who are of maximal educational value to that resident. During this rotation you will function as the neurology expert who co-manages these patients with any of the other disciplines including neurosurgery, trauma, anesthesiology and pulmonary/critical 1 Adapted from 'Neurocritical care for the houseofficer' by A.I. Qureshi, MD, A. Bhardwaj, MD, and J.A. Uletowski, MD, Ph.D., with permission. 2 care medicine. For organizational and educational purposes, orders are typically written by the primary teams unless there are emergent circumstances. Neuro-ICU residents do not take ICU call during this rotation, but patients who require neurological evaluations or follow-up on diagnostic tests after hours need to be signed out to the in-house neurology resident on-call. Similarly, patients who require assessments on the weekend will need to be signed out to the neurology resident on-call, the senior resident on-call, and the Neurocritical care/stroke fellow oncall. Conversely, you should receive a sign-out on new consults, new patients, or new issues regarding these patients from those who were on call overnight (typically the night float) first thing in the morning. Residents rotating on the Neurocritical care service will work Monday – Saturday. On Sunday night, the SR resident should update the Con Neuro-ICU list. Fellows on call for the weekend, please sign out to attending and/or fellow starting service on Monday. We have Neurocritical Care Initial Consult and Neurocritical Care Follow-up notes that are on the EPIC system. For studies, the consent forms are located at the E2 front desk in a file cabinet. If we are out of consent forms or workbooks for any of the studies please page Irina Eyngorn (17032, ext 87333) to replenish the supply. The schedule of the rotation will be as follows: Monday – Friday Schedule 9 AM: Attending Rounds. It is expected that you pre-round on all your assigned patients. This includes gathering vitals, labs, radiology results, and neurodiagnositic study results, asking the RN for any overnight events, patient examination, and formulating a plan for your patient. At times there is a high volume of patients, so make sure you prioritize by seeing our primary patients and/or the most active (sickest) patients first. During rounds order all tests and follow up imaging studies. After rounds: Complete your notes and send to the attending to cosign. There are two medical ICU teams: Blue and Green. You must contact the resident or fellow caring for your patients to update them on our recommendations for the day. The MICU fellow’s phone is ext. 4-8820. The Blue team resident phone is ext. 3-3688. The Green Team phone is ext. 6-8069. Look at the E2 board for patient assignments (Green versus Blue). At times we may join the MICU’s team for rounds to discuss the patients and update them on our recommendations. You also need to contact other services that we are consulting to update them on our assessment and plan. 3 – 4:30 PM: Afternoon rounds on active patients and new patients with attending. You are expected to see the new consults and post-op patients prior to afternoon rounds. (The time may vary on Wednesdays due to the resident lecture series.) 6 PM: Resident sign out: The Neuro-ICU resident is expected to sign out to the on-call resident. Please inform the on-call resident of any potential transfers. If you have clinic please call the fellow for an update on the patients and who needs to be signed out to the resident on-call. 3 Weekends As the Attending and Fellow cover the Stroke ward service and ICU service, the timing of ICU rounds will be attending dependent. On Sunday, the ward Senior resident is expected to preround on the ICU patients with the Fellow on call. Patients followed by the Neurocritical care service: 1. All of our primary stroke and general neurology patients. 2. We cover all consults for patients residing in the E2 and NICU. 3. We cover consults of post-cardiac arrest patients in the CCU. These are the only patients in the CCU that the Neurocritical Care service covers. 4. The post-op neurosurgical patients (Steinberg, Chang, Dodd) and Neuro-IR cases (Marks, Do, Dodd). 5. All head trauma patients (patient name written in red with a green star) 6. Each resident on the ICU service is expected to follow up to 8 patients (cap), please run all new cases by the fellow as the fellow needs to run the service. If there are more than 8 patients for each resident, the fellow will pick up the overflow. ICU Board color codes: If the patients name is written in a particular color that is the primary service. The colored stars adjacent to the patients name represent the consult services following the patient. Every morning the unit assistant will also write on the board a list of the on-call residents and fellows for the various services. Black = Neurocritical care; also referred to as “neuromed” Green = Neurosurgery Blue = MICU (a parenthesis with designated “(B)” or “(G)” represents the Blue or Green MICU team) Red = Trauma Orange = Transplant Phone numbers: MICU fellow phone 4-8820 Green team MICU resident phone 6-8069 Blue Team MICU resident phone 3-3688 JoAnn Ceranski for TCDs and SSEPs, pager 15173 Tim Chamberlain, ICU social worker, pager 16314 California Encephalitis Project 510 307-8808, Fax 510 307-8599 Other phone numbers should be on your laminated card. Weekly Conferences Monday 10:30 – 11:30 am Last Monday of the month is a multidisciplinary critical care conference, Anesthesia conference room, H 3565 4 - 5 pm: Neurovascular conference (optional) 5 - 6 pm: Stroke Conference, Neurology conference room, H 3150 4 For Stroke Conference: the Stroke and Neurocritical Care fellows will each be expected to give Journal club approximately 3 times/year, Difficult Case Conference approximately 3 times/year and present an aspect of their research at the Research Conference 1 time/year. The Fellow will approach a Faculty mentor for each presentation. Journal Club requires a thorough and thoughtful review of a recent high impact paper, ideally one with broad interest to the group (Neurology/Neurosurgery/Neuroradiology). A thorough understanding of the research methodology, as well as the strengths and weaknesses of the study, should be highlighted. Tuesday 12:00pm Neurology Case conference, Neurology conference room, H 3150 Wednesday 2:30 – 6:15pm Neurology Residents’ Lecture Series (Residents will be gone from the ICU) 4 pm Neuroradiology Conference, Neurology conference room, H 3150 Thursday 10:30 -11:30 Critical Care Fellows lecture series, Anesthesia conference room, H 3150 (neurocritical care fellows mandatory) Friday 7 – 8 am Neurosurgery Grand Rounds 8 - 9 AM Neurology Grand Rounds Neurological Examination in the ICU A neurological examination of a critically-ill neurologic patient is directed towards evaluating three major components: 1. Determining the level of consciousness 2. Determining if there is a focal neurologic deficit 3. Evaluation of respiration and hemodynamic status One of the important parts of the neurological examination in the intensive care unit is the assessment of level of consciousness. The neural tracts that mediate consciousness start from central pons and hypothalamus and project signals to the thalamus and cortex. Thalamic relay nuclei send diffuse projections to the cortex. The cortex feeds back on the thalamic nuclei promoting arousal. A depressed level of consciousness can occur from dysfunction of brainstem activating systems or impaired cerebral hemispheres or both. Note any sedating medications that can interfere with level of consciousness and the neurological exam. The examination of the critically-ill neurologic patient should consist of the following components: Higher cortical functions: Glasgow Coma Score Orientation and attention 5 Speech Cranial nerves and brainstem: Midbrain-CN II: Pupils, Visual fields Midbrain-Pons-CN III, IV, VI: Eye movements Pons-CN V: Corneal reflex, facial sensation Lower Pons-CN VII: Facial grimace & movements Lower Pons-CN VIII: Oculocephalic/ oculovestibular reflexes Medulla-CN IX, X; Gag and cough reflex Medulla-CN XI: Shoulder shrug Medulla –CN XII: Tongue movements Motor system: Strength Tone Reflexes, Plantar reflexes Sensory system: Proprioception Touch Pain Temperature Cerebellar system: Finger-to-nose, heel–to-shin testing Coordination Gait Meninges: Neck rigidity The neurological examination is just as important in the unconscious patient and will reveal important diagnostic signs. Below is a chart with helpful exam tips. Exam Info Level of Consciousness Depth of coma is important to determine. Test Call patient’s name loudly. Call to patient from opposite direction pt is looking to see if tracks to voice. GCS (see below). Note: Intubated patients are charted as 1T under verbal. Whenever possible: cognitive skill memory reasoning emotional states Abnormal findings Pupils 6 The efferent limb of the pupillary response is mediated by the parasympathetic fibers that travel with the oculomotor nerve causing pupillary constriction. The response to light is assessed separately for both eyes using a strong penlight, preferably in a dark room. Note the size of the pupils and any asymmetry. Ipsilateral dilation of the pupil with loss of pupillary reflex can be the sign of transtentorial herniation, isolated third nerve palsy or midbrain dysfunction. Eye Movements Observe spontaneous eye movements. Note if the pupils are midline or deviated and conjugate (ie move in parallel) or disconjugate (ie move in separate directions). Eye movements can be observed by calling the attention of the patient to one side and then the other or by attracting their attention with visual stimulus. Elicit the oculocephalic (doll’s eyes) reflex. (Do not do if possible c-spine injury). Briskly turn head side to side or flex and extend. Eyes should move conjugately in the opposite direction the head is turned. Elicit the oculovestibular reflex (cold caloric testing). Ensure the tympanic membrane is intact. Elevate the head 30. Irrigate ear with 30-50 mL cold (33C) water. Wait 5 minutes before doing other side. The sensory component of the reflex is mediated by the trigeminal nerve and the motor component by the facial nerve. Cough Take a wisp of cotton or tip of sterile gauze. Gently touch the sclera and assess for eyelid closure. Alternatively may use artificial tears instead of cotton wisp. Noxious stimulation can be elicited by applying supraorbital pressure. Also observe the face when applying noxious stim to extremities. You may see an asym in the face with grimacing. Facial Corneals Visual fields Open patient’s eyes. Bring one finger towards their eye in each of the visual fields. This should elicit a blink. Sensory and motor component is mediated through the vagal nerve. The cough reflex is usually the last preserved reflex in deteriorating level of consciousness. In intubated patients, a soft suction catheter is advanced through the endotracheal tube. Absent oculocephalic reflex. Eyes will move in same direction as head movement (eg fixed with the motion) and indicate a pons or midbrain dysfunction. If eyes remain fixed this indicates brainstem injury. A normal response to cold water would be a slow deviation toward the stimulated ear and a fast beating nystagmus away from the stimulated ear. No blink to threat. Bilateral loss of corneal reflexes is reflective of poor level of consciousness. Unilateral loss is seen in lesions involving the trigeminal or facial nerve. Asymmetry or weakness. A cough is the normal response which is lost in deeply comatose patients and patients with a brainstem lesion. Sensory Reflexes Motor 7 It is important to recognize that the changes in tone and reflexes after upper motor lesions develop over days and it is not unusual to see flaccidity and hyporeflexic extremities in the acute phase. Apply central noxious stim by applying supraorbital pressure or sternal rub. It is important to assess if there is an asymmetry in reflexes or in the case of GBS to assess if the reflexes are present. Test all reflexes in the arms and legs. Note any asymmetry. Presence of extensor plantar response (Babinski reflex) can be elicited by scratching the bottom of the foot looking for extension movement of the toes. Recognizing sensory deficits helps in differentiating pure motor lesions from sensorymotor lesions, which have different localization in the central nervous system. Determining a sensory level in patients helps localize lesions to the spinal cord. In patients who are unable to follow verbal commands, a limited sensory examination is performed which includes applying a noxious stimuli to each limb and observing for facial grimace and limb withdrawal. In patients who have intact level of consciousness, more detailed sensory testing should be performed. If stimulation produces asymmetric response, limb weakness is present. Note tone and bulk in each extremity. Glasgow coma score Best verbal response 1. No response (Patients that are intubated give a T or a 1) 2. Incomprehensible sounds 3. Inappropriate words 4. Disoriented and converses 5. Fully oriented and converses Best eye response 1. Does not open eyes 2. Opens eyes to painful stimuli 3. Opens eyes to verbal command 4. Opens eyes spontaneously Best motor response 1. No motor response 2. Extension (decerebrate posturing) 3. Flexion (decorticate posturing) 4. Withdraws purposefully from painful stimuli 5. Localizes painful stimuli 6. Follows verbal commands Weakness with increased muscle tone, hyperreflexia, and extensor plantar response suggests an upper motor neuron lesion which can occur at any point in the corticospinal tract including the motor cortex, brainstem, and spinal cord. No facial grimace or withdrawal. 8 SCORE RANGE: 3 – 15 Evaluation of respiration, blood pressure and heart rate: Many neurological diseases are associated with abnormalities in respiration and hemodynamic status. Don’t forget the ABCs with your neurological examination. AIRWAY: Oropharyngeal reflexes associated with decreasing level of consciousness can lead to compromised airway and require an airway adjunct or intubation. BREATHING: Pattern, rate, oxygen saturation and ABGs should be monitored. Neurologically impaired patients are prone to pneumonia from aspiration. Also multiple patterns of respiration can be observed in neurological disease: 1. Hypoventilation consisting of shallow rapid respirations in patients with decreased level of consciousness or neuromuscular disease 2. Cheyne-Stokes breathing consisting of a regular waxing and waning (crescendo – decrescendo) respiration pattern attributed to decreased responsiveness of CO2 receptors in the respiratory centers leading to retention of CO2 followed by rapid respirations to reduce the CO2. The respirations decrease after CO2 is reduced and alternating pattern of hyper-and hypoventilation is observed. This pattern is commonly seen in bihemispheric lesions or in metabolic encephalopathy. 3. Apnea described as episodes of absent respirations seen in infratentorial (brainstem or cerebellar) lesions. It is thought to be secondary to compression of respiratory centers in lower brainstem. 4. CENTRAL hyperventilation is seen in brainstem lesions from tentorial herniation or other injury to the brainstem. However, most often hyperventilation in the comatose patient is secondary to a systemic illness, such as, metabolic acidosis from lactic acidosis, ketoacidosis, uremia, or organic acid poisoning. CIRCULATION: Tachycardia or bradycardia can be the manifestations of acute intracranial process. Supraventricular tachycardias are the most common rhythm disturbances observed in patients with intracranial process. Careful attention should be paid to the neurological status in patients in the neurocritical unit who present with new onset heart rate abnormalities. Other potential etiologies for tachycardias are pain, anxiety or agitation, fever, hypoxemia, and volume depletion. Patients in the neurocritical care unit are monitored continuously using an electrocardiographic monitor. EKG changes occur frequently in patients with CNS disease and do not necessarily indicate coronary ischemia. Blood pressure changes are common in critically ill neurologic patients. Acute hypertension is seen with cerebral ischemia and raised intracranial pressure. It is important to recognize that hypertension represents a protective response to maintain cerebral perfusion in these conditions and should not be treated rapidly into the normal range. Other causes of hypertension include pain and agitation. Hypotension is seen in patients with massive intracranial injury and spinal cord injury due to loss of sympathetic tone which results in peripheral 9 vasodilation. However, attempts should be made to evaluate for other causes of hypotension, such as, sepsis, dehydration, and sedative side effects. BASIC PATHOPHYSIOLOGIC PRINCIPLES Four basic topics will be discussed: 1. Mass effect and cerebral herniations 2. Cerebral edema 3. Intracranial pressure 4. Cerebral perfusion pressure Mass effect and cerebral herniations: The cranium represents a closed compartment. The contents of the cranium consist of 80% brain tissue, 10% cerebrospinal fluid, and 10% blood. Due to lack of expansible properties, small increases in the volume can lead to large increase in intracranial pressure (ICP). Initial increase in mass effect in any compartment is compensated by displacement of CSF to the spinal canal and with increasing pressure may lead to obliteration of sulci and ventricles (if the ventricles are not obstructed/hydrocephalus). Further increase in volume in one compartment will push the normal contents into the other compartments within the cranium, a phenomenon known as herniation. The main forms of herniations are: 1. Subfalcine herniation: Increase in mass effect in one hemisphere pushes the cingulate gyrus and the anterior cerebral artery to the other side under the falx (a vertical fibrous sheet that separates the upper part of both hemispheres). Patients usually present with decreased level of consciousness and sometimes with unilateral or bilateral anterior cerebral artery infarction with prominent leg weakness. 2. Transtentorial herniation: Large increase in mass effect can lead to downward shift of a hemisphere through the tentorium (a crescent shaped fibrous sheet that separates the hemispheres from the cerebellum). The uncus is the part of the hemisphere that herniates initially and compresses the ipsilateral third nerve leading to pupillary dilatation (uncal herniation). Further expansion compresses the corticospinal tracts in the cerebral peduncles in the midbrain leading to contralateral hemiparesis. 3. Central herniation: Bilateral vertical shift (hydrocephalus) or diffuse parenchymal swelling can lead to herniation of both hemispheres causing bilateral pupillary dilatation, loss of consciousness and quadriparesis. 4. Transforamenal herniation: Increase in mass effect in the brainstem and cerebellar region can lead to protrusion of the cerebellar tonsils through the foramen magnum. Initial presentation includes apnea and cardiovascular compromise due to direct compression of respiratory and cardiovascular centers in the medulla. Cerebral Edema: Cerebral edema represents an important component of mass effect from any cerebral lesion. There are three types of cerebral edema: 10 Vasogenic edema: This represents excess of extracellular fluid in the brain as a result of breakdown of the blood-brain barrier. This form of edema is seen with tumors and abscesses and is responsive to steroid treatment. Cytotoxic edema: Due to direct cellular damage, the transport system of the cell membrane is impaired leading to accumulation of water within the cells. This type of edema is seen in cerebral infarction. Interstitial edema: Extravasation of fluid through the ventricular ependymal layering into the parenchyma due to hydrocephalus. This type of edema has minimal mass effect and responds to ventricular CSF drainage. Intracranial pressure: The pressure within the cranial vault is known as intracranial pressure (ICP). Normal values are between 5 – 15 mm Hg (10 – 20 cm H2O; 1cm H2O = 0.7 mmHg). A diagram of the pressure – volume relationship can be seen below. Note that eventually small changes in volume create large changes in pressure. ICP greater than 20mm Hg is taken as the threshold for starting treatment to reduce ICP. The ICU monitors show the ICP in mmHg. The ICP can be measured in the subarachnoid space, ventricular cavity and the brain parenchyma by using an external ventricular drain (EVD) or Camino (fiber optic) bolt. An EVD is placed in the lateral ventricle through the skull, frontal lobe and towards the Foramen of Monro (channel between the lateral ventricles and the third ventricle). The catheter is capable of measuring ICP as well as draining CSF, but not at the same time. The nurse can assist you with transducing the ICP and ensure the transducer is in the correct location. The Camino device is a fiber optic device that is placed over the brain parenchyma to measure ICP. Intracranial Pressure - Volume relationship Pressure Low compliance 20 mm Hg High compliance Volume 11 Cerebral perfusion pressure (CPP): CPP is a function of both systemic blood pressure and intracranial pressure. It is estimated by the equation CPP = MAP (mean arterial pressure) - ICP. In a healthy adult with normal ICP, the CPP is going to approximately equal the MAP. The cerebral arterioles maintain constant cerebral blood flow (CBF) by altering their diameter with changing systemic MAP/CPP, a phenomenon known as autoregulation. Under normal autoregulatory conditions, CBF is preserved within a wide range of systemic MAPs (range of 60 to 150 mm Hg)/ CPPs (see diagram below). However, conditions that elevate ICP usually impair the normal autoregulatory capacity and in diseased states the cerebral blood flow may become dependent on the CPP in more of a linear fashion (see dotted line below). Cerebral ischemia may result if there is not adequate perfusion pressure. Therefore, maintaining adequate cerebral blood flow requires reduction of ICP and at times augmentation of the perfusion pressure by increasing the systemic blood pressure. It is generally recommended to keep the CPP above 70 mm Hg. Cerebral Perfusion Pressure and Autoregulation Cerebral Blood Flow Cerebral Perfusion Pressure/MAP BASIC TREATMENT PRINCIPLES The following topics are discussed: 1. Treatment of raised ICP 2. Mechanical ventilation 3. Blood pressure management 4. Fluid status management 5. Nutrition 6. Sedation Treatment of raised ICP : 12 The following treatment modalities are available for management of raised intracranial pressure: 1. Keep the head of bed elevated and the head midline: Keep the HOB greater than 30 degrees and keep the head midline to promote venous return and to prevent compression of the jugular vein. 2. Mannitol: Mannitol is an osmotic agent that is thought to lower ICP by increasing serum tonicity and drawing edema away from the injured brain parenchyma, which reduces the cerebral water content and therefore reduces ICP. Furthermore, mannitol decreases the viscosity of blood by decreasing the Hct, which may increase cerebral blood flow and O2 delivery to the brain. The initial dose is 0.5-1.0g/kg and maintenance dose is 0.25g/kg every 4 to 6 hours to keep serum osmolality elevated (300 - 320 mOsm/L). There are concerns that serum osmolality above 320 may lead to kidney injury. Serum osmolality is monitored frequently when repeated doses of mannitol are used. 3. 3% saline: (Note: higher concentration, 23% saline can be used. This is given by the physician for the theoretical concern of cerebral demylination that is seen in children.) Boluses of 3%NaCl may be used to decrease edema. It may also be necessary to use 3% to treat hyponatremia related to cerebral salt wasting. 4. CSF drainage: ICP is reduced by decreasing the ventricular volume in patients with hydrocephalus. Drainage of CSF from the ventricular cavities also creates a gradient for movement of extracellular fluid from the edematous regions into the ventricles and subsequent drainage. 5. Hyperventilation: Artificial hyperventilation to reduce PaCO2 to 25-30mm Hg is ONLY used for acute treatment of raised ICP. Lowering the PaCO2 induces local alkalosis in the extracellular spaces of cerebral tissue leading to vasoconstriction of the cerebral arterioles. This reduces the blood volume in the cranial vault and reduces the ICP. The effect is transient (6-24 hours) and not without consequences (cerebral ischemia), therefore hyperventilation should ONLY be used for acute emergent situations and not as a prophylactic measure. The potential for inducing cerebral ischemia with hyperventilation has been well described. 6. Pentobarbital: Pentobarbital reduces cerebral metabolism, thereby reducing cerebral blood flow (volume), and consequently lowers the ICP. The dose is a 5 – 20 mg/kg loading dose, followed by a maintenance dose of 1- 3 mg/kg/hour. The cerebral activity is monitored by continuous electroencephalography (EEG) and the goal is to induce burst suppression where there is occasional spontaneous electrical activity followed by suppression of baseline EEG rhythm. Pentobarbital is a cardiovascular and respiratory depressant and requires intensive cardiovascular monitoring and mechanical ventilation. It should be used cautiously with IV fluids and vasopressor medications readily available to maintain pressure. Pentobarbital also reduces gastric motility and impairs immune function. Due to the complications associated with pentobarbital coma and the lack of evidence that it improves patient outcome, its usage should be reserved for those patients who are refractory to standard therapies to lower ICP. 7. Steroids: Steroids reduce vasogenic edema and therefore reduce ICP. However, steroids have only demonstrated benefit in vasogenic edema due to neoplasm and have a long onset of action and therefore have a limited use in acute management of raised ICP. Mechanical ventilation: There are three important reasons for intubation and mechanical ventilation in the critically ill neurologic patient. 13 1. Airway protection due to poor level of consciousness or poor oropharyngeal strength. Serial assessment of level of consciousness and cough reflex can provide a useful index for planning extubation. In the absence of any improvement, these patients require tracheostomy and chronic mechanical ventilation. 2. Decreased or absent ventilatory drive due to brainstem injury. In the absence of any improvement, these patients require tracheostomy and chronic mechanical ventilation. 3. Decreased ventilatory strength due to neuromuscular disease. PaCO2 retention (PaCo2 > 50 and pH < 7.35), routine forced vital capacities (VC < 15-20 mL/kg), negative inspiratory force (nif < - 25 – 30 cmH2O), and patient comfort (or discomfort) are the best guides to adequacy of ventilatory support. (See more on this topic in the section for GBS and Myasthenia crisis.) In the acute phase of the disease, these patients may be most comfortable on assist control (AC) mechanical ventilation as they get support with every breath taken. Patients with reversible neuromuscular conditions such as Guillain-Barre syndrome and myasthenia gravis can be weaned from ventilation. The duration of weaning is variable depending upon the individual’s general health and severity of the disease. Forced vital capacity and negative inspiratory force are measured daily to assess any improvement and to guide the weaning process. A day of observation on minimal support is recommended prior to extubation to observe for fatigue. Blood pressure management: The goal of BP management is to maintain adequate cerebral perfusion pressure (CPP = MAP – ICP). The MAP goal in the critically ill neurologic patient is usually higher than required for systemic perfusion. The lower limit of the blood pressure goals should be adequate to keep the CPP greater than 70mm Hg (or at least greater than 60 mm Hg in head trauma patients). Any hypotension should be aggressively treated. The agents that may be used to increase systemic blood pressure include: intravenous fluids, colloid (albumin), and pressors, such as, phenylephrine, or ionotropic agents. Acute hypertension in patients with intracranial disease can be a protective response to either raised intracranial pressure or cerebral ischemia. Lowering blood pressure in such situations can worsen cerebral blood flow. If blood pressure needs to be reduced, some of the following agents may be used: Antihypertensive options: Nicardipine – calcium channel blocker. Arteriodilator and thus does not decrease preload. Avoid in patients with severe aortic stenosis. (Nicardipine gtt 5 – 15 mg/hour) Nitrates and nitroprusside - can lead to cerebral vasodilation and increase the ICP and these drugs are usually avoided. Sublingual nifedipine can lower the blood pressure precipitously and cause cerebral ischemia and is also avoided. (Nipride gtt range 0.1 – 10 micrograms/kg/min) Labetalol – a beta blocker. Alpha1, beta1, beta2 receptor blocker. A first line anti-hypertensive agent. Bradycardia may necessitate another agent. (Labetatol 5 – 20 mg IV q 15 min, max dose 300mg/day) Metoprolol – a beta blocker. Beta 1 receptor selective blocker. (Metoprolol 5 – 10 mg IV q 4 – 6 hours, can via NGT for HTN may start at 25mg BID and increase as tolerated) Esmolol – a beta blocker. Beta 1 receptor selective blocker. Esmolol has more heart rate lowering effects, than BP lowering effects. (Esmolol gtt range 25 – 300 micrograms/kg/min) 14 Enalapril – ACE I. Side effects include angioedema and worsening kidney function - watch creatinine. (Enalapril 0.625 – 5 mg IV q 6 hours) Hydralazine - a direct acting vasodilator, which may also increase ICP. (Hydralazine 5 – 20 mg IV q 4 – 6 hours) Fluid status management: The optimal fluid status for most patients with neurological disease is euvolemia. Systemic dehydration does not reduce cerebral edema but may worsen cerebral perfusion. Therefore adequate fluid intake should be provided for all patients. In healthy adults, sufficient fluid is required to balance gastrointestinal losses of 100-200ml/day, insensible losses of 5001000ml/day through cutaneous and respiratory route and urinary losses of 1000ml/day. Average maintenance fluid is 1ml/kg/hour (~ 2000-2500ml/day for adults). Most patients require greater amount of fluids in the ICU due to losses from tachypnea, fever, and the use of osmotic agents like mannitol, which can lead to significant diuresis. Normal saline is the intravenous fluid of choice. Hypotonic solutions can worsen cerebral edema and should not be used. Glucose containing solutions are avoided as well because of the concern of hyperglycemia causing further brain injury. Keep in mind that many patients in the ICU are often receiving a number of IV medications and medication gtts. These medications in combination with their tube feeds may be sufficient fluid intake without the addition of maintenance fluids. Nutrition: Nutrition intake should be started within 24-48 hours as long as the patient is hemodynamically stable. Due to decreased level of consciousness, most patients require a nasogastric tube for administration of nutritional preparations. Ileus and delayed gastric emptying are common occurrence in the neurocritical care unit. Reglan intravenously 5 - 10mg every 4 - 6 hours may be required for large gastric residuals. Erythromycin is an alternative agent and it does not have the neurological side effects associated with reglan (AMS, NMS). Constipation is associated with head and/or spinal trauma. Laxative should be used for prophylaxis against constipation. Gastrointestinal motility is also worsened by narcotics, barbiturates, and systemic infections. Sometimes, intravenous nutrition may be required. Sedation: Sedation is required for patients who are a danger to themselves or others because of confusion or agitation. Sedation may also be necessary in intubated patients for comfort or in patients who are manifesting hyperadrenergic symptoms due to drug or alcohol withdrawal. The sedation regimen should preserve the neurological examination as best possible, or have the potential to be discontinued with rapid return of an uncompromised examination. Increasing confusion and agitation may be manifestations of increasing ICP or a new cerebral lesion; therefore a careful assessment prior to administration of any sedation is required. Patients that are sedated for intubation comfort should have their sedation regimen discontinued at least once a day. The four most common agents used in the ICU for sedation and analgesic purposes are: benozodiazepines, narcotics, haloperidol, and propofol. Midalozam is a short acting benzodiazepine which is used in bolus doses of 0.02-0.08mg/kg or as an infusion at 0.050.1mg/Kg/hour. Midalozam reduces the cerebral metabolism without significantly altering the cerebral blood flow. Fentanyl is a synthetic opiate with rapid distribution and short half-life. The 15 usual dose for bolus is 0.25-1.5ug/Kg and infusion is 0.3-1.5ug/Kg/hour. Major side effects include respiratory depression and hypotension. Naloxone is used to reverse the adverse effects of opiates if required. Haloperidol is a butyrophenone antipsychotic agent, which has minimal effect on respiratory and cardiovascular status. The usual dose is 0.01-0.05mg/Kg. Propofol is an ultra-short acting alkyl phenol. Its clinical action on cerebral activity and intracranial dynamics is similar to short-acting barbiturates. The usual dose is 0.1-0.3mg/Kg for boluses and 0.66.0mg/Kg/hour for infusion. Propofol’s side effects include: respiratory depression, hypotension, hypertriglyceridemia, pancreatitis, propofol infusion syndrome (fever, rhabdo). Propofol should be discontinued if the patient develops severe hypertriglyceridemia, pancreatitis, or propofol infusion syndrome. Propofol should only be used in intubated patients. TREATMENT OF SPECIFIC GROUPS OF CRITICALLY ILL NEUROLOGIC PATIENTS Head Trauma The primary injury in head trauma is hemorrhagic with extradural, subdural, or parenchymal involvement; and a non-hemorrhagic component consisting of cellular swelling, vasogenic edema, contusions and diffuse axonal injury. Diffuse axonal injury results from the shear stress induced by rapid acceleration/deceleration of the neural tissue. In its severe form, hemorrhagic foci in the corpus callosum and dorsolateral rostral brain stem with microscopic evidence of diffuse injury to the axons (axonal retraction balls, microglial stars, and degeneration of white matter tracts) are observed. It can present as protracted loss of consciousness immediately following head trauma in the absence of any mass occupying lesions on CT scan. Secondary injury commonly results from hypotension or hypoxia. The management of head trauma is based on the following principles: 1. Support vital signs by managing the airway and normalizing blood pressure and heart rhythm. Patients with GCS of 8 or below should be intubated. The PaO2 should be maintained above 100mmHg (95-105mmHg) and the initial MAP goal is 90mmHg or above. Once ICP recordings are available, the CPP should be kept above 70mmHg (at least above 60mmHg). Intravenous fluids and vasopressors (Phenylephrine or Dopamine gtts) may be required to support blood pressure. 2. An ICP monitor preferably a ventriculostomy should be placed in patients with GCS of 8 or less to guide treatment. A BOLT may also be used to monitor pressure post head injury. Hyperventilation may be used to reduce PaCO2 to 25-30 mm Hg only IF there is a clear evidence of increased ICP either on the basis of ICP monitors recordings or clinical examination including unilateral or bilateral pupillary dilatation with loss of light reflex and decreased level of consciousness. Mannitol therapy should be initiated concomitantly. Hyperventilation is then weaned and in the absence of high ICP, PaCO2 is kept between 3545 mm Hg. 3. Surgical evacuation of extradural, subdural, or intracerebral hematomas is considered. The size of the lesion and the clinical status determine eligibility for surgical evacuation. 16 4. Anticonvulsants should be considered on a prophylactic basis. Phenytoin and carbamazepine have demonstrated efficacy in preventing acute post traumatic seizures. Phenytoin (20mg/Kg) is loaded intravenously at a rate less than 50 mg/min and then 100mg every 8 hours for maintenance. 5. Steroids have no demonstrated efficacy in head trauma and are avoided. 6. The ICP rise is most prominent on day 3 due to coalescence of small hemorrhages and worsening cerebral edema. ICP greater than 20mm Hg should be treated with mannitol or hypertonic saline. If ICP does not respond to repeated doses of mannitol or serum osmolality is greater than 310mOsm/l, then consider either pentobarbital coma, decompressive surgery, or possibly hypothermia should be considered. Factors that exacerbate ICP such as fever, agitation, hypotension, hypovolemia, hypoxemia and high PaCO2 are aggressively treated. A second rise in ICP is seen in some patients at day 10 again requiring standard ICP treatment. 7. At two weeks, if patients are unable to protect their airway due to poor functional outcome, tracheostomy and percutaneous gastrostomy tube placement are considered in preparation for subacute or chronic care. Other Resources: 1. Critical Care / Neurosurgical Practice Guidelines: http://www.braintrauma.org 2. Critical Care Guideline and Practice Parameters: Society of Critical Care Medicine: http://sccmwww.sccm.org/professional_resources/guidelines/index.asp 3. Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries: Spine Universe: http://www.spineuniverse.com/displayarticle.php/article2081.html 4. Guidelines for the Management of Mild Traumatic Brain Injury: Eastern Association for the Surgery of Trauma (EAST): http://www.east.org/tpg.asp 5. Guidelines for the Management of Penetrating Brain Injury: American Association of Neurological Surgeons; Neurosurgery://on Call: http://www.neurosurgery.org/sections/section.aspx?Section=TR Intracerebral hemorrhage Non-traumatic intracerebral hemorrhage can present with acute onset of loss of consciousness and focal deficits. Most spontaneous ICHs are due to underlying hypertension. The diagnosis is made on a noncontrast CT scan. The management principles are described below: 1. Airway support. Patients with GCS of 8 or less are usually intubated. 2. Blood pressure control. Most patients are hypertensive at presentation as a consequence of pre-existing hypertension and raised intracranial pressure. Hematomas continue to expand in the first few hours after onset in many patients. High blood pressure may predispose to expansion of hematomas. Blood pressure is reduced if MAP is greater than 140-150mm Hg for more than 15 minutes according to the recommendations of National Stroke Association. The American Heart Association Guidelines 2007 state that there is little evidence to support any specific recommendation but have the following comments with regard to BP: “Isolated SBP less than 210 mmHg is not clearly related to hemorrhagic expansion or to neurological worsening. Reduction in MAP by 15% (mean MAP 142 to mean MAP 119) does not result in 17 3. 4. 5. 6. 7. 8. CBF reduction in humans as measured by PET. In one prospective observational study, reduction of SBP to a target of SBP < 160/90 was associated with neurological deterioration in 7% patients and hemorrhagic expansion in 9% but was associated with a trend to improved outcome in those patients whose SBP were lowered within 6 hours from onset. Baseline blood pressure was not associated with growth of the hematoma in the largest prospective study of ICH growth and in the Recombinant Activated Factor VII Intracerebral Trial. Hemorrhagic enlargement occurs more frequently in patients with elevated SBP, but it is not known whether this is an effect of increased growth of ICH with associated increases in ICP or a contributing cause to the growth of the hematoma. Rapid decline in blood pressure during acute hospitalization was associated with increased death rate in one retrospective study. Experience in traumatic brain hemorrhage, as well as in spontaneous ICH, support preserving the CPP > 60 mmHg. With the above in mind, the AHA 2007 suggested guideline for treating elevated BP in ICH are: If the SBP > 200 or MAP > 150, recommend continuous BP monitoring at least q 5 min and IV continuous infusion of BP lowering medication. If the SBP >180 or MAP > 130 and there is evidence of or suspicion of increased ICP, then consider ICP monitoring and reducing the BP such that the CPP is maintained 60 – 80 mmHg. If the SBP >180 or MAP >130 and there is no evidence or suspicion of elevated ICP, consider modest BP reduction to target a MAP <110 or BP < 160/90 using medication and following BP q 15 mins.” Blood pressure should not be too aggressively treated particularly in the absence of knowledge regarding ICP. The hematoma induces a zone of ischemia around itself by compresion of microvasculature in the surrounding tissue. Lowering the blood pressure precipitously can worsen tissue damage due to ischemia. Coagulation profile, which includes prothrombin time, INR, activated partial thromboplastin time, and platelet count. Patients who have abnormal coagulation profile may require fresh frozen plasma or platelet replacement to prevent expansion of hematoma. Patients who have blood in the ventricles are at risk for developing obstructive hydrocephalus as the blood clot prevents drainage of CSF. A ventricular catheter is inserted in patients with hydrocephalus. Evacuation of clot is recommended if cerebellar hemorrhages threaten transforamenal herniation and compression of medulla. Evacuation of hematoma in other sites is dependent upon the clinical condition and size of the clot. Many patients have pre-existing hypertension. Oral hypertensive therapy should be held in the first 48 hours due to long half-life and unpredictable response in presence of elevated ICP. Blood pressure should be managed by intravenous antihypertensive therapy with short half lives and titrated to response. Oral antihypertensive agents can be started after 48 hours if the patient’s condition is stabilizing. You may wish to start with shorter acting antihypertensive agents and titrate up as needed. Elevated ICP can be seen in ICH and is result of mass effect of the hematoma and cerebral edema around the hematoma. Cerebral edema is at its maximum 48 hours after the ICH and starts to recede after 5 days. However, some patients may have their maximum edema at 2-3 weeks following ICH. Standard measures of ICP control may be required. Some patients have a poor recovery and will require tracheostomy and gastric tube for supportive care. Patients who have poor level of consciousness at presentation and have a large hematoma on CT scan usually have poor recovery. 18 Reference: Broderick J, et al. AHA/ASA Guidelines for the Management of Spontaneous Intracerebral hemorrhage in Adults 2007 Update. Stroke 2007; 38:2001-2023. Subarachnoid hemorrhage Subarachnoid hemorrhage (SAH) is a blood clot in the subarachnoid space most prominent at the base of the brain. Most subarachnoid hemorrhages are the result of a ruptured cerebral aneurysm. The diagnosis is made on the CT scan. In cases where clinical picture is very suggestive of SAH and the CT scan is negative (10%), a lumbar puncture is performed to look for blood and xanthochromia (color due to breakdown products of bilirubin) in the CSF. Once the diagnosis is established the following management is recommended: 1. Aggressive control of BP to avoid rebleeding of the aneurysm keeping in mind the CPP goals. Control of headache with acetaminophen/or narcotics. Patients can be sedated if they are anxious with low dose benzodiazepines or haloperidol. Nimodipine (60 mg po every 4 hours) is initiated for prophylaxis of cerebral vasospasm. Some physicians use steroids (decadron 4 mg every 6 hours) to reduce chemical meningitis associated with subarachnoid hemorrhage. 2. A four vessel cerebral angiography is performed as early as possible to diagnose the aneurysm. About 20% of patients may have multiple aneurysms. A CT angiogram of the head and neck may be done on admission to find the aneurysm. 3. Surgical clipping or coiling of the aneurysm is performed early to prevent rebleeding. Steroid taper may be used post-operatively by the NS service to alleviate swelling due to mechanical manipulation of the brain. 4. Patients are at risk for cerebral vasospasm following SAH. The exact mechanism in which vasospasm occurs is not known. One theory is that oxyhemoglobin released from the breakdown of clot induces constriction of the cerebral blood vessels. The reduction in CBF due to vasospasm is compensated by collateral circulation. Cerebral vasospasm occurs in two-thirds of the patients 3-10 days after SAH. Cerebral ischemia (clinical vasospasm) is seen in only half of the patients with vasospasm. It may manifest as focal deficit, decreased level of consciousness, or a combination of both. The onset can be acute or insidious over 24 hours. Daily transcranial Doppler ultrasound is performed for early detection of cerebral vasospasm. This non-invasive test measures blood flow velocities in the proximal intracranial arteries. Increased velocities signify cerebral vasospasm. Once the aneurysm is secured, modest hypervolemia (central venous pressure [CVP] of 5-8 mm Hg) is maintained to prevent cerebral ischemia from vasospasm. If cerebral ischemia occurs due to vasospasm, hypervolemia and hypertension is instituted. Hypervolemia (CVP of 8-12 mm Hg or pulmonary capillary wedge pressure of 12-14mm Hg) is achieved by intravenous administration of normal saline at a rate of 150-200cc per hour. Intermittent bolus of normal saline or colloids may be required to achieve the goal. Hypertension is achieved by administration of phenylephrine or ionotropic infusion to increase the mean arterial pressure (100-140mm Hg). If there is no response within 4 hours, therapies such as intra-arterial nicardipine infusion (other stroke centers use other intra-arterial agents like verapamil) and angioplasty are considered. Once the clinical symptoms have improved, the hypertensive/hypervolemic therapy is weaned over 48 hours under close observation for recurrence of cerebral ischemia. 19 5. Obstructive hydrocephalus can result from blood in the ventricles or subarachnoid blood obstructing the arachnoid villi (where CSF is absorbed into the venous sinuses). The symptoms consist of decreased level of consciousness. Immediate treatment is insertion of ventricular catheter. The blood disolves over time and the hydrocephalus can resolve. However, sometimes, fibrosis develops with the arachnoid villi and a ventriculo-peritoneal shunt may be required for chronic CSF diversion. 6. Any neurological deterioration is aggressively investigated by a CT scan to rule out hydrocephalus. CSF and serum analysis is performed to rule out meningitis and electrolyte disturbances, respectively. Cerebral angiography may be required for the diagnosis of vasospasm. 7. Steroids if used are quickly tapered over a few days. Hypervolemic therapy is titrated against TCD velocities and neurological examination by the end of second week. Nimodipine is continued for 21 days after onset of SAH. 8. Anticonvulsant therapy should be initiated in all patients suspected of seizure or with seizures. In one study phenytoin was associated with worse cognitive outcomes in SAH patients. Other agents, while unstudied, may be considered in patients with SAH and seizures. Cerebral Ischemic Infarction Stroke is the third leading cause of death in the United States. Ischemic strokes account for ~85% of strokes (~15% being from ICH/SAH). Cerebral ischemia and/or infarctions result from thrombotic or embolic occlusion of an artery supplying the brain. Thrombus can form in the small arteries of the brain causing a stroke (small vessel disease). Thrombus can also form in the large arteries supplying the brain resulting in narrowing of the artery and hypoperfusion to the brain. Emboli can originate from a proximal artery (like an atherosclerotic plaque in the carotid artery or aorta) causing artery to artery emboli. Emboli can also come from the cardiac valves or the left heart(cardioembolic stroke). Middle cerebral artery infarction may consist of the following symptoms: hemiparesis, hemisensory loss, aphasia (L) or neglect (R), and gaze deviation. Vertebrobasilar artery infarction may present as a combination of decreased level of consciousness, cranial nerve involvement, and cerebellar dysfunction. Infarction may not be visualized on CT scan up to 24 hours, therefore, diagnosis is made clinically on most occasions or by magnetic resonance imaging (Diffusion Weighted Imaging (DWI): acute ischemia may be seen on DWI within minutes to ~ 2 weeks later). Occasionally you may appreciate a hyperdense MCA sign or an insular ribbon sign (subtle loss of the gray-white differentiation) on head CT. Management is based on the following principles: 1. Airway management. Patients with vertebrobasilar ischemia are at risk for respiratory compromise due to a decreased level of consciousness, central apnea, and/or poor airway control due to CN injury and loss of protective reflexes. Intubation is considered early for these reasons. Patients with MCA infarction may develop decreased level of consciousness when cerebral edema is maximum (2-5 days after onset) and may require intubation at that point. 2. Blood pressure management. It is widely accepted that elevated BP in cerebral infarction is a response to preserve cerebral blood flow. Blood pressure should only be reduced if it is 20 3. 4. 5. 6. 7. greater than 220/120 over 15 minutes according to the recommendations of the National Stroke Association. Aggressive lowering of BP is avoided unless signs of hypertensive encephalopathy develop. Oral antihypertensive agents should be started at the end of the first week. Intravenous tissue plasminogen activator administration has been shown to improve outcome if given within 3 hours of symptom onset and when the NINDS/FDA inclusion and exclusion criteria are followed. Theoretically, tPA lyses the clot and restores blood flow in the affected distribution. The dose is 0.9mg/Kg, of which 10% is given as a bolus followed by an infusion over 1 hour. The greatest risk with tPA is hemorrhagic transformation (~6% symptomatic intracranial hemorrhage rate in NINDS trial). After the lysis of clot, the blood flow returns and enters the parenchyma through the disrupted blood-brain barrier. It is recommended to keep the blood pressure < 180/105 after IV tPA. For patients who are ineligible or fail IV tPA, mechanical thrombectomy (MERCI retrieval device) may be considered in the 0 – 8 hour window after symptom onset and in patients with substantial neurological deficit (NIHSS > 8). Intra-arterial therapy for acute stroke is not approved by the FDA, but may be considered in patients with MCA occlusions who can be treated within the 3 - 6 hour window after symptom onset and who have little CT abnormality. Given the high mortality associated with carotid T (intracranial ICA bifurcation) occlusions and basilar artery occlusions, IA therapy could be considered for this population as well. Studies have demonstrated that the combination of IV therapy with IA therapy is feasible, but studies are ongoing to determine its efficacy. Present studies have shown that there is viable tissue within the periphery of the ischemic zone. This tissue is partially ischemic and is very sensitive to potential secondary neuronal injuries such as hyperglycemia and hyperpyrexia. Therefore, strict glucose and temperature control should be instituted in the acute phase of the infarction. There is no evidence to support urgent anticoagulation to prevent stroke recurrence or stroke extension in patients with acute stroke. Patients with venous infarct secondary to sinus thrombosis should be anticoagulated. Patients with cardioembolic strokes from atrial fibrillation or severe cardiomyopathy (depressed EF) should be eventually anticoagulated. One must consider the size of the infarct and the risk of hemorrhagic transformation as well. One may also consider anticoagulation in patients with fluctuating vertebral-basilar symptoms and severe arterial stenosis. We have a neurocritical care heparin protocol order set. Please ask the unit assistant for a copy if you need it. Our lab follows anti-heparin levels (therapeutic goal 0.3 – 0.5) and not PTTs. Patients who are not anticoagulation candidates are started on an antiplatelet agent on presentation. Aspirin therapy within 48 hours of stroke onset has been shown to be of benefit and is recommended that acute stroke patients receive aspirin therapy. In patients who receive IV tPA, antiplatelet therapy should be started 24 hours after thrombolytic therapy. Depending on the etiology of the stroke, other antiplatelet agents are preferred to aspirin for secondary stroke prevention in TIA or noncardioembolic strokes. (Aggrenox ASA 25/ Dipyridamole ER 200 PO BID (grade 2A recommendation); side effects include headache and may want to start with once a day administration for the first few days. Clopidogrel (Plavix) 75 mg PO QD (Grade 2B recommendation). Cerebral edema seen in cerebral infarction is predominantly cytotoxic with a less prominent vasogenic component. It peaks at 48-72 hours and starts resolving by 5 days. Cerebral edema can cause life threatening mass effect (herniation). Surgical decompression with 21 hemicraniectomy may be necessary. A recent meta-analysis concluded that in younger patients (18 – 60 y/o) with a malignant MCA infarction, early decompressive therapy (< 48 hours) reduces case fatality by 50% with a modified Rankin Scale score ≤ 3 (0 = no symptoms to 6 = death, mRS 3 means can walk independently) at one year in 55% of the survivors. However, the decision to perform decompressive therapy should be on a case by case basis. Standard treatment for edema (osmotic therapy) is instituted as necessary. 8. Echocardiography and cerebral angiography may be performed early to determine the underlying etiology of cerebral infarction. Significant carotid stenosis, which is narrowing greater that 70% of unaffected segment, should be evaluated for possible carotid endarterectomy or stenting. References: 1. Albers GW, et al. Antithrombotic therapy and Thrombolytic therapy for ischemic stroke_7 th ACCP Conference. Chest 2004; 126(3):483S-512S.(new updated version just came out Chest 2008; 133:630-669.) 2. TPA for Acute Ischemic Stroke. The NINDS and stroke rTPA study group. NEJM 1995; 333:1581-7. 3. CAST (Chinese Acute Stroke Trial). Randomized placebo-controlled trial of the early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet 1997;349:1641-9. 4. The International Stroke Trial (IST). A randomized trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischaemic stroke. Lancet 1997; 349:1569-81. 5. ESPS2. J Neurol Sci 1996; 348:1329-1339. 6. CAPRIE. Lancet 1996; 348:1329-39. 7. Smith W et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005; 36:1432-8 8. Vahedi K, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of the three randomized controlled trials. Lancet 2007; 6:215-22. Status Epilepticus Status epilepticus affects around 150,000 people in the US every year and the mortality is almost 30%. It is defined as persistent seizures, either intermittent or continuous, that result in unconsciousness for a period of time. The time used to be defined as 30 min, but you should start treating a patient who is seizing according to the algorithm below as soon as you come into contact with them. Time is brain! How to treat a patient in status is diagrammed below (from Lowenstein, 2006). Note that the third line agent (what you use after lorezepam and fosphenytoin) is variable. His paper goes over the evidence. Basically, phenobarb coma works the best but has the highest number of side effects, so it is reasonable to start with propofol if you want – it causes less hypotension and it is usually close at hand in the ICU and the ED. If a patient doesn’t start waking up from seizures once they stop overtly shaking, an EEG is indicated – almost half of patients with persistent coma after status are having nonconvulsive seizures. You can expect that about 40% of patients will not respond at all to the first two drugs, and 82% will continue to have some seizures after correct loading with lorezepam and fosphenytoin. Once you start with a third-line agent, or a fourth, also consider asking the epilepsy service for advice. 22 In terms of diagnosing the problem, below are some meds and etiologies to think about (From Khaled and Hirsch, 2007). Don’t forget ABCs (ie, intubate them and make sure they are oxygenating well), electrolytes, a head CT (followed later by MRI but you need to know quickly if they have an acute neurosurgical emergency), and your LP. LP is indicated in any patient with a fever, history c/w encephalitis or if there is no known cause of seizures. As soon as you decide to LP the patient, you need to write for acyclovir and cover for bacterial meningitis. If the lumbar puncture is negative, you can then D/C those meds. Be sure to get a careful opening pressure and several extra tubes, for add on labs if you have abnormal glu, protein, or cells. 23 24 Finally, there are non-neurologic complications of status: tachyarrythmia, hyperpyrexia, rhabdomyolysis, and pulmonary edema. Lorezepam and fosphenytoin can cause mild hypotension, and fosphenytoin can cause cardiac arrythmias. Propofol (to a lesser degree) and pentobarbital (to a high degree) are direct suppressors of cardiac function and may require the use of pressors. Pentobarb also causes gastric stasis and immunosuppression. Regardless of meds, patients are at high risk of aspiration pneumonia and bacteremia. References 1. Holtkamp, M., 2007. The anesthetic and intensive care of status epilepticus. Curr Opin in Neurology 20:188-193. 2. Khaled, K.J.A. and Hirsch, L., 2007. Advances in the management of seizures and status epilepticus in critically ill patients. Crit Care Clin 22:637-659. 3. Lowenstein, D., 2006. The management of refractory status epilepticus: An update. Epilepsia 47(Supl 1):35-40. 25 Guillain-Barre syndrome Guillain-Barre syndrome (GBS) is a demyelinating disease of peripheral nerves, which progresses over 2-4 weeks to maximum severity. Variant axonal forms include acute motor axonal neuropathy (AMAN) and acute motor and sensory axonal neuropathy (AMSAN). Clinical presentation includes acute quadriparesis (more prominent in the lower extremities) with or without cranial nerve involvement, respiratory failure and autonomic involvement. Common preceding illnesses include acute gastroenteritis (Campylobacter jejuni) or flu-like illness (Mycoplasma pneumonia, Cytomegalovirus, Epstein Barr virus). Diagnosis is made on the basis of clinical presentation (weakness, areflexia or hyporeflexia) and CSF analysis (elevated protein in the absence of leukocytosis: albumino-cytologic dissociation). Nerve conduction studies (NCS) are helpful in diagnosing demyelination (earliest is prolonged distal and F-wave latencies; reduced conduction velocities and conduction block occur later). If NCS are normal early, then they should be repeated 1-2 weeks later. Severe cases of GBS are due to extensive demyelination or axonal damage which is reflected by decreased CMAP (compound muscle action potential) and EMG changes of denervation. Antibodies to gangliosides (GM1, GD1a and GQ1B), stool culture and serology for C.jejuni, acute and convalescent sera for CMV, EBV and M.pneumoniae can also be tested. Critical care issues include respiratory failure and autonomic dysfunction. The management is based on the following principles: 1. Respiratory support. Patients with either bulbar dysfunction (even in the presence of normal respiratory function) or respiratory failure (mechanical failure) need respiratory support. The decision to intubate is made on the basis of clinical evaluation and pulmonary function tests. Clinical evaluation includes assessment of the strength of cough and sniff. Patients can be asked to count loudly as long as possible on one breath. Normal persons can count easily to 30, while patients who count less than 20 have significant impairment of respiratory muscles. Tachypnea, forehead sweating, tachycardia, paradoxical respiration, episodic use of accessory muscles of respiration, staccato speech, impaired mentation and air hunger indicate impending respiratory failure. Signs of bulbar weakness include nasal voice, poor palate mobility while saying “ah”, abolished or decreased gag reflex. Predictors of intubation include time from onset to admission of less than 7 days, inability to cough, inability to stand, inability to flex head, lift shoulders and arms and vital capacity of < 60% predicted. Forced vital capacity (FVC) and negative inspiratory force (NIF) is measured every 4 to 6 hours. FVC is defined as measured volume with forceful exhalation after maximal inhalation. NIF is defined as maximum inspiratory pressure generated after maximal sucking in through a mouth piece while occluding the nose. Patients who have FVC<15ml/Kg and NIF< -25 cm H2O are usually intubated. Also, a drop in VC of >55% from supine to sitting position is a better indicator of diaphragmatic weakness than isolated VC measurements. Due to concomitant facial weakness, it may be difficult to establish a tight seal on the mouth piece for such ventilatory parameters. Therefore, parameter results are interpreted in conjunction with clinical evaluation. Hypoxemia and atelectasis are also indicators of neuromuscular failure (hypercarbia occurs later). 26 Endotracheal intubation is more difficult in GBS patients due to exaggeration of dysautonomia (arrhythmias, hypotension) and potential for lethal hyperkalemia if succinylcholine is used. Topical airway anesthesia, atropine and IV sedation may be all that is required. Once the patient is intubated, full support is advised for the early part of mechanical ventilation. Assist control or IMV modes in the acute phase of the disease offer complete rest for the respiratory muscles. The adequacy of ventilatory support is determined by patient comfort and lack of CO2 retention. After the acute phase, the patient can be placed on CPAP and pressure support can be titrated down as tolerated. Daily FVC and NIF are measured to follow improvement in respiratory muscle strength. Patients are likely to be extubated if FVC and NIF are greater than 15 ml/Kg and - 25 cm H2O, respectively. The pulmonary function (PF) score can be calculated by adding the values of VC, NIF and Maximal expiratory pressure. A PF ratio is obtained by dividing the PF scores from days 1 and 12 after intubation. A PF ratio of < 1 indicates need for prolonged intubation. The decision to place a tracheostomy may be postponed for 2 weeks. If there is no improvement of PFT’s from baseline, tracheostomy is performed (percutaneous is preferred). If PFT’s improve from baseline, an additional week can be considered for weaning attempts. Early tracheostomy should be performed in patients with axonal form of GBS. 2. Immunotherapy: Treatment of acute demyelination requires administration of either immunoglobulin (IVIG) or plasma exchange (PE). The utility of PE or IVIG in treating the axonal form of GBS is unknown Intravenous Immunoglobulins: IVIG is recommended for patients who require aid to walk within 2 weeks of onset of neuropathic symptoms (level A) or 4 weeks of onset of neuropathic symptoms (level B).IVIG is as effective as PE and is easier to administer, but relapses may be more common than with PE. The dose is 2.0 gm/Kg total over 3 to 5 days. Complications include renal impairment, aseptic meningitis, hay fever like syndrome, anaphylaxis, congestive heart failure, DVT and hyperviscosity syndrome. Plasma exchange: PE is usually given in one plasma volume exchange (each 40-50 ml/Kg) every other day (total 200-250 ml/kg) for a total of 5 exchanges via a central venous catheter. 5% Albumin or Fresh frozen plasma (FFP) is used as replacement fluid. Coagulation parameters (PT, PTT, fibrinogen, hemoglobin) and calcium should be monitored while using FFP or Albumin respectively. Complications include catheter related problems (i.e. bleeding, infection, DVT, pneumothorax), fluid overload (pulmonary edema), hypotension, electrolyte imbalance and arrhythmias. PE is recommended for non-ambulant patients within 4 weeks of onset (level A, class 2) and for ambulant patients within 2 weeks of onset (level B). The effects of IVIG and PE are equivalent. Sequential treatment: Treatment with PE followed by IVIG does not have superior effect than either treatment administered alone (level A, class 1), but numbers in the studies were small and a small treatment benefit could have been missed. Steroids: Corticosteroids are not recommended for treatment of patients with GBS (level A, Class 1), as they worsen outcome. 27 3. Autonomic lability: This includes tachycardia with occasional arrhythmias, severe bradycardia, asystole, blood pressure fluctuations, ileus and SIADH. These are common in patients with advanced generalized weakness and respiratory failure and can be precipitated by drugs, endotracheal suction etc. Monitoring of pulse and BP is recommended until ventilator support is discontinued. Pharmacological treatment is avoided initially due to the transient and unpredictable nature of the episodes. If symptoms necessitate treatment, standard short acting cardiac medications are effective. Rarely, temporary pacemaker is needed. 4. Pain: Pain is an important component of GBS and is usually located in the back and shoulder regions and is worse at night. Deep, achy pain located in the back and legs responds well to re-positoning, massage and compresses. Narcotics are used if required. Sometimes when constipation and urinary retention become an issue due to narcotic use and autonomic dysfunction, intravenous toradol can be used. Neuropathic pain occurs in the extremities. Longer acting and less addictive medication like tricyclic antidepressants, non steroidal antiinflammatory agents, and anticonvulsants (gabapentin, carbamazepine) are used chronically with some effect. 5. Supportive care: DVT prevention: Subcutaneous unfractionated heparin or LMWH and support stockings/SCD’s (avoid covering fibular head) are recommended till patients are able to walk independently. Bowel and bladder care: Daily abdominal auscultation and monitoring of opioid administration is recommended. Erythromycin and metoclopramide can be used to treat ileus (contraindicated in patients with dysautonomia). Stool softeners and GI prophylaxis are essential. Bladder drainage (continuous closed system or sterile intermittent catheterization) is recommended. Rehabilitative Therapy: Gentle strengthening involving isometric, isotonic, isokinetic and manual resistive and progressive resistive exercises are recommended. Proper limb positioning (to avoid contractures and pressure palsies), posture and orthotics. Nutrition: GBS is a hypercatabolic state. Continuous enteral feeding with high calorie, high protein formula is recommended. Mood: Exercise program may be beneficial. Careful use of antidepressants without aggravating dysautonomia (SSRIs) is also recommended. Anxiety is treated with benzodiazepines or buspirone. Patients with GBS are virtually “locked in”. Communicating with the patient via sign, pictorial board or computer communication devices is essential, as is explaining daily care plan to the patient. Suggested Reading: 1. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barre syndrome: a systematic review. Brain. 2007.(epub) 2. Hughes RA, Cornblath DR. Guillain-Barre syndrome. Lancet. 2005; 366:1653-66. 3. Hughes RA, Wijdicks EF, Benson E, Cornblath DR, Hahn AF, Meythaler JM, Sladky JT, Barohn RJ, Stevens JC; Multidisciplinary Consensus Group. Supportive care for patients with guillain-barre syndrome. Arch Neurol. 2005 ; 62:1194-1198. 28 4. Hughes RA, Wijdicks EF, Barohn R, Benson E, Cornblath DR, Hahn AF, Meythaler JM, Miller RG, Sladky JT, Stevens JC; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barre syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003 Sep 23; 61:736-740. 5. Chalela JA. Pearls and pitfalls in the intensive care management of Guillain-Barré syndrome. Semin Neurol. 2001; 21:399-405. 6. Lawn ND, Wijdicks EF. Post-intubation pulmonary function test in Guillain-Barré syndrome. Muscle Nerve. 2000; 23:613-616. Myasthenic Crisis Myasthenia gravis (MG) is an autoimmune disease in which autoantibodies directed against the acetyl-choline or muscle specific tyrosine kinase receptor (MuSK) at the neuromuscular junction lead to muscle weakness. Only motor strength is affected without involvement of sensation or reflexes. Myasthenic crisis: Acute weakness of the respiratory and/or bulbar muscles due to MG is known as myasthenic crisis. It is defined by an acute episode of respiratory muscle weakness (vital capacity (VC) is < 1L, NIF (negative inspiratory force) < - 20 cm H20, or requirement of mechanical ventilation.) It is usually precipitated by infections, initiation of steroids, or change in medication. Clinical presentation is typically one of two types: 1.) respiratory distress with excessive effort of the respiratory muscles; or 2.) in severe muscle weakness, the patient is hypercarbic with poor effort and somnolence. Cholinergic crisis: Acute or chronic overdose of anticholinesterase medications can cause muscle weakness mimicking myasthenic crisis. Other signs of cholinergic excess (e.g increased secretions, fasiculations, bradycardia) are present. This is best excluded by temporarily stopping anticholinesterase medications while the patient is presumptively treated for the myasthenic crisis. Treatment is supportive. The management is based on the following principles: 1. Respiratory support. The respiratory failure in myasthenic crisis does not improve quickly and the patients progressively get worse due to fatigue. Therefore, intubation with mechanical support should be instituted early in the episode. Most patients with MG are young and therefore can maintain good oxygen saturation even with impending respiratory failure. Monitoring VC and NIF (measures of inspiratory muscles) should be done every 1-4 hours. VC of < 15 ml/kg, NIF of < 20 cm H2O and PEF (positive expiratory force) of < 40 cm H2O are indicators for intubation. Clinical state, use of accessory muscles and air hunger are also reliable clinical indicators of impending failure. Assessment of strength of sniff, cough and counting to 30 (measures of expiratory muscle function) can also be used. Respiratory failure in MG can also occur from upper airway muscle dysfunction (i.e. bulbar weakness) with pooling of secretions, upper airway collapse and aspiration. Examining the head and neck muscles is a surrogate way of assessing upper airway function. Bulbar weakness is evidenced by hoarse voice, dysarthria, dysphagia with nasal regurgitation, chewing fatigue, 29 facial weakness, jaw drop, neck flexor or extensor weakness. In such situations, VC and NIF may be normal. BIPAP(bi-level positive airway pressure) can be used initially and seems to be successful in preventing intubation especially in patients without hypercapnia of > 50 mm Hg. Disadvantages include aspiration risk due to lack of airway protection. Patients should be fully supported in the early part of mechanical ventilation and most physicians would use assist control or IMV with minimal sedation in the acute phase of the disease to completely rest the respiratory muscles. The adequacy of ventilatory support is determined by patient comfort and lack of CO2 retention. Oropharyngeal secretions can be a problem due to the cholinergic effect of anticholinesterase agents used in the treatment of myasthenia. Oral glycopyrrolate (1mg every 6 hours) can be used to reduce these secretions. Patients are at risk to develop atelectasis due to poor inflation of lungs and require sighs, PEEP(positive end expiratory pressure) on the ventilator with postural drainage of the affected lungs if atelectases develop. Weaning attempts should begin when a) overall strength is improving b) VC > 10 ml/kg c) NIF > -20 cm H2O d) oxygen requirement is 40% or less e) no medical or infectious complications. The initial goal is to eliminate mandatory ventilation (i.e. cut down on rate) and then wean PS (pressure support). If patient is able to tolerate PS of 8-15 cm H2O for a day, gradually decrease PS by 1-2 cm H2O every day. Weaning trials are preferred during earlier part of the day. Daily FVC and NIF are measured to follow improvement in respiratory muscle strength. Patients are likely to be extubated if FVC and NIF are greater than 15ml/Kg and 25cm H2O, respectively. Predictors of prolonged intubation include age > 50, pre-intubation serum bicarbonate ≥ 30 mg/dl and VC < 25 ml/kg within 6 days of initial intubation Tracheostomy is usually unnecessary because duration of intubation is frequently < 2 weeks. However, reintubation may become necessary due to the erratic nature of the illness. 2. Immunotherapy: Treatment of myasthenic crisis requires either plasma exchange (PE) or intravenous immunoglobin (IVIG) administration. PE removes the antibodies in the serum and improves clinical symptoms. Usually five exchanges are performed on alternate days. Each exchange is 1 plasma volume (i.e. 50 ml/kg). (For more details, refer to GBS treatment). No RCT exist for PE in MG. Improvement is seen within a few days, but lasts only for few weeks. Small studies suggest that PE produces much quicker response in myasthenic crisis as compared to IVIG, although both are equally efficacious. IVIG can be used in patients if PE is not feasible. Immunoglobins reduce production of other immunoglobins such as anticholinergic antibodies due to negative feedback. They also bind to antibodies neutralizing their antigen binding capacity. The usual dose is 0.4gm/Kg/day intravenously for 5 days. Response may take up to 2-3 weeks. 3. Steroid: High dose steroid treatment which includes methyl prednisolone 100mg every 8 hours or oral prednisone 80 mg a day for three days followed by a taper to maintainance dosage is used to improve acute symptoms of myasthenia. 4. Anticholinesterase medications: Rule out cholinergic crisis. One approach is to stop anticholinesterase medications in the acute phase and re-start them orally when patients are stronger and about to be extubated. Another approach is using IV pyridostigmine infusion at 1-2 mg/hr during crisis. (1 mg IV = 30 mg oral). Watch out for fatal arrhythmias and excess respiratory secretions. 30 5. Re-booting of immune system with G-CSF/Cyclophosphamide: Anecdotal reports document using extremely high doses (50 mg/kg/day IV for 4 days of cyclophosphamide followed by GCSF) in refractory patients (especially with MuSK antibodies). 6. Thymectomy: A multicenter randomized trial is underway. It should not be attempted without minimizing patient weakness first and is rarely used during hospitalization for myasthenic crisis. 7. Other measures: include treatment of infections (most common cause of myasthenic crisis), and removal of medications that exacerbate myasthenia (aminoglycosides, anti-arrhythmics, and magnesium). 8. Supportive care: refer to GBS treatment. Suggested reading: 1. Ahmed S, Kirmani JF, Janjua N, Alkawi A, Khatri I, Yahia AM, Souyah N, Qureshi AI. An Update on Myasthenic Crisis. Curr Treat Options Neurol. 2005;7: 129-141. 2. Juel VC. Myasthenia gravis: management of myasthenic crisis and perioperative care. Semin Neurol. 2004 ;24: 75-81. 3. Rabinstein AA. Update on respiratory management of critically ill neurologic patients. Curr Neurol Neurosci Rep. 2005;5: 476-482. Spinal Cord Trauma Acute spinal cord injury has an initial component of contusion and intraparenchymal or extradural hemorrhage. This is followed by release of prostaglandins from the breakdown of cellular membranes. These prostaglandin derivatives lead to vasoconstriction and secondary ischemia in the local tissue. The acute management is based on the following principles: 1. All patients who present within 8 hours of injury should receive high dose steroid (methylprednislone) which includes a bolus dose of 30 mg/Kg followed by an infusion of 5.4 mg/Kg/hour for 23 hours. High dose steroids reduce the release and activation of prostaglandin metabolites and presumably reduce secondary injury. Blood pressure is usually augmented using vasopressors to increase perfusion of the spinal cord for the first 24 hours. 2. Patients who have high cervical injury (damage to phrenic nerve) require mechanical ventilation in the absence of other complications. In the initial phase of spinal cord injury, there is vasodilation and hypotension due to loss of sympathetic innervation. This is particularly prominent in lesion above the 6th thoracic level because the innervation of the splanchnic vessels come out at that level. Aggressive intravenous fluid and vasopressors may be required during this period to maintain adequate blood pressure. However, this vasodilatory phase is followed by increased sympathetic tone and vasoconstriction due to loss of higher control over a period of 48 to 72 hours. This period can be associated with autonomic dysfunction such as marked rise in blood pressure in response to sympathetic stimulation by pain, urinary bladder distension, or constipation. Intravenous vasodilators, general anesthetics, or regional blockage of neural impulses with spinal or epidural anesthesia may be necessary. 31 Infections of the nervous system Bacterial meningitis (community acquired and nosocomial): Clinical presentation: Bacterial meningitis is a neurological emergency. The triad of fever, altered mentation and neck rigidity has a low sensitivity (44%). However, the absence of any of these symptoms rules out meningitis with 99-100% sensitivity. 95% of patients will have at least 2 of the following 4 symptoms: headache, fever, neck stiffness and altered mental status. Fever may be lacking in the elderly, immunocompromised or in patients with prior antibiotic treatment. Complications include seizures (independent predictor of mortality), hydrocephalus, stroke (due to vasculitis), venous thrombosis, abscess, cerebral edema and herniation. Indications for ICU admission are: age > 60, altered mentation, papilledema or imaging evidence of high ICP, focal deficits/deterioration despite appropriate antibiotics, seizures, respiratory failure and shock. Organisms: S.pneumoniae (penicillin resistant strains), N. meningitidis (children and young adults), Group B streptococcus, Listeria monocytogenes (>50 yrs and immunocompromised) and H. influenza (older adults) are the most common bugs in community acquired meningitis. Nosocomial infections are mostly gram negative (pseudomonas, klebsiella etc). In head trauma/CSF leak or post-neurosurgical patients, S. aureus and gram negative bacilli are common organisms. Diagnosis: CSF analysis should be obtained ASAP, even without prior CT. Exceptions are patients with the following clinical features(age > 60, abnormal language or inability to follow 2 commands, seizure in past week, visual field abnormalities, arm or leg drift, gaze or facial palsy) or with suspected mass lesion/ raised ICP ( immunosuppression, coma, focal deficits, seizures, papilledema, poorly reactive pupils, ocular palsies, bradycardia, irregular respirations, sedation or pharmacologically paralyzed patient). A 22 or 25 gauge needle should be used and smallest amount of CSF should be removed. Diagnostic sensitivity does not decrease even up to 2 hours after antibiotic initiation. CSF usually shows > 100 WBC/mm3 with neutrophilic predominance, low glucose and elevated protein. CSF/serum glucose ratio of < 0.4 is sensitive and specific. CSF protein is most resistant to change with antibiotic treatment and remains elevated for more than 10 days. In post-op neurosurgical patients, CSF lactate cut-off of 4 mmol/L is more sensitive than CSF/serum glucose ratio for diagnosis of bacterial meningitis. Blood cultures also should be drawn. PCR, CSF counterimmunoelectrophoresis, CSF latex agglutination are also done. Serum procalcitonin drawn at admission and at 48 hours can be used to monitor response to treatment, replacing repeat LP (which is done at 48-72 hours after treatment initiation). CT/MRI is used to detect complications including abscess, stroke, sinus thrombosis and also source of infection (i.e. sinusitis, mastoiditis etc). Treatment: Antibiotics: A delay of > 3 hours in starting antibiotic treatment is independently associated with 3 month mortality. Initial treatment is broad, and started with intravenous ceftriaxone 2gm every 12 hours or cefotaxime 2gm every 6 hours plus vancomycin 15 mg/kg IV every 8-12 hours (adjust further dosing based on trough levels). In older adults (>50 years), ampicillin (2gm every 4 hours) is added to the above regimen to cover for S. agalactiae and Listeria moncytogenes. In patients with impaired cellular immunity, ampicillin, vancomycin and a broad spectrum cephalosporin such as ceftazidime (2 gm IV every 8 hrs or cefepime IV every 8 hours) is used for empiric therapy. In patients with recent head trauma or neurosurgery, broad spectrum antibiotics effective against both gram-positive and gram-negative should be given such 32 as vancomycin and ceftazidime/cefepime. Any source of infection (hardware) should be removed. If the gram stain of the CSF is suggestive of S. pneumoniae, a second agent such as vancomycin or rifampicin (900-1200 mg/day IV in 2-3 divided doses) is added due to the high incidence of penicillin-resistant S. pneumoniae. The treatment is continued until the organism in CSF is identified and then antibiotics can be adjusted based on the antibiotic-susceptibility of the organism. If no clinical response is seen within 72 hours of initiating treatment, a repeat lumbar puncture is performed and antibiotics are changed. The antibiotic treatment is continued for 10-14 days in S. pneumoniae meningitis and 7 days in H. influenzae or N. meningitidis meningitis. Infection with L. monocytogenes and gram-negative bacilli should be treated for 14-21 days. Steroids: Steroid use is advocated by some for reducing long-term consequences of inflammation in the subarachnoid space such as deafness and hydrocephalus in patients who have high concentration of bacteria in the CSF (bacteria visible on gram stain of CSF), S. pneumoniae and evidence of elevated ICP. Dexamethasone (0.15mg/Kg every 6 hours for 4 days, 1st dose at the same time as antibiotic or just before) is the recommended treatment. It is not recommended for patients previously treated with antibiotics, nosocomial, post neurosurgical or shunt related meningitis. For patients with TB or HIV meningitis, total of 4-8 weeks (2-4 weeks IV with oral taper) of steroids is advocated. Supportive care: Fluid management (isotonic saline for resuscitation in shock). Prophylactic AED’s are not indicated. Meningitis in immunocompromised patients: Immunocompromised patients (e.g. HIV, post transplant, cancer) are susceptible to opportunistic bacterial, fungal and parasitic infections. HIV patients are at risk for Toxoplasmosis and Cryptococcus and other fungal infections (candida etc). When CD4 count < 50, toxoplasma, nocardia and aspergillus (focal lesions), Cryptococcus and CMV (diffuse lesions) are culprits. TB meningitis should also be considered. Cryptococcal meningitis presents with HA, nausea, somnolence or obtundation, fever, neck stiffness and cranial nerve palsies. CSF shows high opening pressure, low glucose, high protein with normal (in case of severe immunosuppression) to elevated lymphocytic cell count. CSF india ink stain identifies the capsule. Serology for Cryptococcal Antigen is very sensitive and relatively specific for CNS involvement. Treatment is with 6 weeks of fluconazole (200-400 mg/day) or amphotericin B (0.4-0.6 mg/day). Toxoplasmosis presents as ring enhancing lesions on MRI with focal deficits. PCR is 100% sensitive for toxoplasmosis. It is treated with sulfadiazine and pyrimethamine (along with folinic acid). Optimal treatment of fungal infections is ill-defined. Most have an induction phase of 2-6 weeks with an amphotericin based regimen, to which flucytosine can be added. Liposomal amphotericin is used in patients with renal insufficiency. Intrathecal amphotericin (.01 to 1.5 mg) at daily-weekly intervals with hydrocortisone (15-25 mg) can be used for patients who relapse or who do not respond to systemic therapy. Voriconazole is later used for chronic suppressive therapy, as it has good CNS penetration, especially for aspergillosis. Routine use of caspofungin is not recommended due to poor CNS penetration. Viral encephalitis: Clinical presentation includes fever, change in mental status and seizures. A flu-like prodrome can be identified. Most common viruses include herpes simplex (HSV), cytomegalovirus 33 (CMV), varicella zoster, mumps and enteroviruses. Recently, West Nile virus is identified as a causative agent. Presence of focal deficits is suggestive of HSV encephalitis. Prodrome of HA and fever followed by altered consciousness, memory loss, personality change, confusion or olfactory hallucinations and seizures is seen. CSF profile consists of lymphocytosis with elevated protein and normal glucose. MRI can reveal diffuse involvement of the brain parenchyma particularly the temporal lobes in HSV encephalitis. Intravenous acylclovir is started when there is a suspicion of encephalitis. The dose is 10 mg/Kg every 8 hours and treatment is continued for two weeks. Dose is renally adjusted. The presence of HSV in the CSF is confirmed by PCR (1/3 rd remain positive for weeks after antiviral treatment). Viral cultures take up to 2 weeks and have little impact on treatment. Other antiviral agents effective against HSV to be considered in case of acyclovir resistance are arabinoside and foscarnet. West Nile virus usually affects persons over age 55. It presents with either movement disorders or with acute flaccid limb paralysis (like polio). Mortality is usually caused by respiratory failure. There is no proven therapy, although ribavarin and interferon alpha 2 b have been tried. CMV encephalitis is treated with ganciclovir (5mg/kg IV every 12 hours) and foscarnet (90mg/kg IV every 12 hours). Cerebral abscess: Clinical presentation includes fever, change in level of consciousness, and focal deficits. The diagnosis can be made by CT scan or MRI. They are often polymicrobial. The most common organism is streptococcus pneumoniae. There is a superimposed anaerobic infection in the necrotic abscess as well. Immunocompromised patients are at risk for fungal, Nocardia and Toxoplasma abscesses. The first line of treatment is intravenous antibiotics consisting of vancomycin (to cover MRSA), ceftriaxone (or cefotaxime) and metronidazole (500 mg every 6 hours) for four-six weeks. Patients with recent neurosurgery should have ceftazidime or cefepime in addition. Surgical aspiration is avoided due to the risk of dissemination of infection through the aspiration track. If clinical symptoms do not resolve after 1 week of treatment and the abscess is unchanged or larger on follow-up CT scan, then surgical evacuation is considered. Nocardia is treated with Bactrim. Post transplant patients should have amphotericin added for suspected fungal etiology. Ventricular catheter infections: There is a 10% risk of meningitis associated with placement of ventricular catheter or Ventriculo-peritoneal (VP) shunts. Risk factors include hemorrhagic CSF, craniofacial fractures, manipulation of catheter under non-sterile techniques, CSF leakage around catheter site, immunocompromised status and longer duration of EVD placement. Risk is highest in the initial days after EVD placement with maximal risk at day 6. In V-P shunts, highest infection rate is up to one month after placement. Most common organisms are Staphylococcus epidermidis (coagulase-negative) and Staphylococcus aureus (in patients not on antibiotic prophylaxis). Gram negative rods are associated with long hospital stay, antibiotic prophylaxis and with V-P shunts. Meningitis presents as fever and change in level of consciousness. Diagnosis can be made by CSF aspirated through the ventricular catheter. Positive CSF culture with CSF pleocytosis (>11 WBC/mm 3, with > 50% neutrophils) and corresponding clinical symptoms (fever > 37.5 C, altered mental status, systemic leucocytosis, CSF/plasma glucose ratio < 0.5) is classified as “definite” catheter related infection. 34 Positive CSF cultures, negative Gram stain with normal CSF cell counts are “contaminants’; positive CSF cultures plus CSF pleocytosis but no clinical symptoms are “suspected’ infections. These apply to patients who are not on prophylactic antibiotics. The treatment is with intravenous vancomycin (1gm every 12 hours) or oxacillin (2gm every 4 hours) plus an anti-pseudomonal cephalosporin (cefepime or ceftazidime). Infected hardware should be removed, replaced or externalized as appropriate. Intrathecal/intraventricular administration of vancomycin (10-20 mg/day), gentamicin and tobramycin (5-10 mg) can also be used (not FDA approved) and continued for a few days after CSF cultures become sterile. Most physicians would start prophylactic antibiotics using cefazolin (1gm every 8 hours) or oxacillin (2gm every 6 hours) treat as long as the ventricular catheter is in place. The use of antibiotic coated EVD’s (with rifampin and minocycline) reduces infection and this effect is maintained even after 10 days of placement. Suggested Reading: 1. Ziai WC, Lewin JJ. Advances in the management of central nervous system infections in the ICU. Crit Care Clin. 2006;22:661-694. 2. Rincon F, Badjatia N. Central nervous system infections in the neurointensive care unit. Curr Treat Options Neurol. 2006 ;8:135-144. 3. van de Beek D, de Gans J, Tunkel AR, Wijdicks EF. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006; 5;354:44-53 35 Additional References and Suggested Reading 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. Tietjen CS, Hurn PD, Ulatowski JA, Kirsh JR. Treatment modalities for hypertensive patients with intracranial pathology. Crit Care Med 1996;24: 311-22. Guidelines for the management of severe traumatic brain injury. Brain Trauma Foundation, American Association of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care. www.braintrauma.org Diringer MN. Intracerebral hemorhhage: pathophysiology and management. Crit Care Med 1993;21: 1591-603 Qureshi Al, Frankel MR. Recognition and management of subarachnoid hemorrhage. Heart Disease and Stroke 1994;3:270-74. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue Plasminogen Activator for acute ischemic stroke. N Engl J Med 1995;333:1581-7 Van der Meche FG, Schmitz PI. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barre syndrome. Dutch Guillain-Barre Study Group. N Engl J Med 1992;326:1123-9 Mayer SA. Intensive care of the myasthenic patients. Neurology 1997;48(suppl 5):S70-S75 Braken MB, Shepard MJ, Collins WF, et al. A randomized controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med 1990;322:1405-11 Quagliarello VJ, Scheld WM. Treatment of bacterial meningitis. N Engl J Med 1997;336:708-16 Mirski MA, Muffelman B, Ulatowski JA, Hanley DF. Sedation for the critically ill neurologic patients. Crit Care Med 1995;23:2038-53 National stroke Associations Consensus Statement. Stoke: the first six hours. Emergency evaluation and treatment. Stroke Clin Updates 1993;4:3-12 Videen TO, Zazulia AR, Manno EM, Derdeyn CP, Adams RE, Diringer MN, Powers WJ. Mannitol bolus preferentially shrinks non-infarcted brain in patients with ischemic stroke. Neurology 2001;57:2120-2 Diringer MN, Edwards DF. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med 2001;29:635-40 Frank JI. Large hemispheric infarction, deterioration, and intracranial pressure. Neurology 1995;45:1286-90 Van gijn J, Rinkel GJE. Subarachnoid hemorrhage: diagnosis, causes and management. Barin 2001;124:249-78 Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 2002;43:146-53 de Gans J, van de Beek D; European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators. Dexamethasone in adults with bacterial meningitis. N Engl J Med 2002;347:1549-56 The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549-56