Here
advertisement

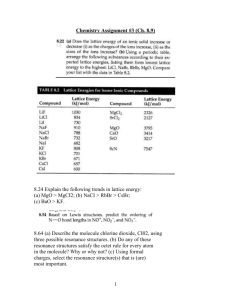
Physical Chemistry Laboratory Exercise Calculating Bond Enthalpies for Diatomic Molecules Electronic structure theory can be used to predict thermochemical data for molecules and chemical reactions. Computer programs have been developed which use ab initio methods to evaluate the energy of a chemical system. One such program is Gaussian 98. A bond enthalpy is defined as the change in enthalpy for the reaction of forming a bond from its constituent atoms. In other words, to calculate the bond enthalpy of a diatomic molecule, one needs to evaluate the absolute enthalpy of the molecule and also the absolute enthalpies of the atoms making up the molecule. The difference in enthalpy between the molecule and its atoms is the bond enthalpy: Hbond H AB H A HB Gaussian 98 could be thought of as an enthalpy meter. You can provide a molecular structure and it will evaluate thermochemical properties such as enthalpy. It does so by solving Schrodinger’s equation within the framework of some approximate model. There are hundreds of different approximate models available and it takes a good working knowledge of these to be able to effectively apply the computer program in solving a real problem. In this exercise, we will evaluate three different approximate models. These happen to be three of the most accurate models available in the program and therefore are the most compute intensive. Since we are dealing with only diatomic molecules, the speed of the calculation is of little concern to us. The three models that we will use are: G1 G2 G2MP2 Consult the “Exploring Chemistry” reference book for more details on these methods. For now, you only need to realize that these are automated procedures within Gaussian 98 which will produce a summary table in the end showing the final enthalpy. In fact, if you specify G2 as the model, the program will compute the G1 and G2MP2 models as well (since the information needed for these is a subset of what is needed for G2). Each member of the class will choose a different diatomic molecule from the list below. For the final analysis, you will need to share your results so that each student has data for the entire set. Cl-Al H-Li Cl-Cl H-Na C-O Li-Na H-F Na-Na Gaussian Input The essential input for any Gaussian job consists of three main pieces of information: 1. 2. 3. Theoretical Model Charge and Multiplicity Atomic Coordinates Theoretical Model You will specify “G2” as the theoretical model. This will request a series of calculations and in the end a summary table will be given. Included in this will be the absolute enthalpy for G1, G2, and G2MP2 models. If you are using the PC, the theoretical model is specified by typing in the word “G2” on the route line. If you are using GaussView on the Silicon Graphics, the G2 model is available as a menu item. Charge and Multiplicity The program needs to know how many electrons are in your system and how these are spinning. It can not guess at this, you must provide this information. For instance if you request a calculation on methane (CH4), it needs to know if you mean neutral methane (no charge, total spin of zero) or methane cation (+1 charge, total spin of ½). The multiplicity can be computed from the total spin as (2S +1). As an example, a calculation on a neutral nitrogen atom would have a charge of zero and a multiplicity of 4. There are three unpaired electrons in nitrogen and so S=3/2. The charge and multiplicity are entered in the appropriate place on the PC version. The charge comes first then the multiplicity (separated by a space or comma). Atomic Coordinates These specify the molecule being calculated. The program will optimize the coordinates according to the model being used, but the use must specify some starting coordinates. For an atomic calculation, one needs only to specify the atom (it will place it at the origin). For a diatomic molecule one only needs to specify the initial distance between the two atoms. If you are using GaussView, the molecule can be simply sketched in. If you are using Gaussian on the PC, you will need to fill in the molecular specification. Here are some examples: For the molecule FB: F B 0.0 0.0 For the atom Br: 0.0 0.0 0.0 1.5 Br 0.0 0.0 0.0 The first symbol represents the atomic symbol. The three floating point numbers (and they must be floating point numbers – including the decimal point) represent the x, y, and z coordinates of the atom. Procedure and Analysis 1. 2. 3. 4. 5. 6. 7. 8. 9. Use Gaussian 98 to compute the absolute enthalpy of each of the atoms in your molecule. Record in your notebook the G1, G2, and G2MP2 enthalpies. Use Gaussian 98 to compute the absolute enthalpy of the diatomic. Record in your notebook the G1, G2, and G2MP2 enthalpies. Also note the final optimized bond distance. Calculate the bond enthalpy for each model and convert to kJ/mol (the IUPAC standard unit). Gather the calculated bond enthalpies for the other diatomics from your classmates. Look up the experimental bond enthalpies (a good online source can be found - see the course home page for a link to Web Elements Database). Prepare a plot of calculated vrs. Experimental bond enthalpies for the series. (Three plots in all, one for each model). What is the value of R squared for each? Which is the best model? Compute the absolute deviation from experiment for each molecule. Use this set of numbers and compute the average (this is called the mean absolute deviation). Compute also the standard deviation for this set of absolute deviations. Comment on which molecules deviate more. Do you have a theory on why Gaussian does not do well on these? Finally, prepare a plot of bond enthalpy showing the trends across the series. Plot the three models as well as the experimental values. Does Gaussian do well at predicting the trends?