TPJ_3866_sm_Appendix
advertisement

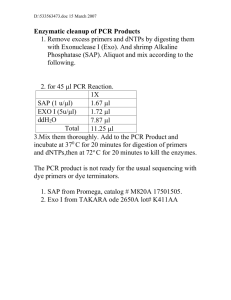
1 SUPPLEMENTARY INFORMATION 2 3 ATHB4, a regulator of shade avoidance, modulates hormone response in 4 Arabidopsis seedlings. 5 6 Céline Sorin1†, Mercè Salla-Martret1†, Jordi Bou-Torrent1, Irma Roig-Villanova1, 7 and Jaime F Martínez-García1,2 8 1. Centre for Research in Agricultural Genomics (CSIC-IRTA-UAB), c. Jordi 9 Girona, 18-26, 08034-Barcelona, Spain; 2. Institució Catalana de Recerca i 10 Estudis Avançats, Passeig Lluís Companys 23, 08010-Barcelona, Spain. 11 †These authors contributed equally to this paper 12 13 Supplementary results 14 15 As an initial approach to study the function of ATHB4, transgenic lines 16 constitutively overexpressing ATHB4 (P35S:ATHB4) were produced. Adult plants 17 segregating from the resulting T1 lines with a single T-DNA insertion displayed 18 three phenotypes in terms of rosette size: wild-type-like (azygous), small 19 (hemizygous) and dwarf (homozygous) (Figure S1a; data not shown). Because 20 homozygous plants were mostly unfertile, the ATHB4-overexpressing lines 21 could only be propagated in heterozygosis. Descendent seedlings from 22 heterozygous plants showed cotyledons with a range of phenotypes, from wild- 23 type to narrow (wt and mut, respectively, in Figure S1) that correlated with the 24 absence or presence of the transgene, respectively, estimated by PCR 25 analyses (data not shown). Based on this character two groups of seedlings 1 1 were visually selected and different traits were measured. The most obvious 2 phenotypes of ATHB4-overexpressing seedlings grown under continuous white 3 light (W) were long hypocotyls and narrow cotyledons (Figures S1c, d). In the 4 strongest lines, cotyledon longitudinal expansion was also reduced, and in older 5 stages primary leaves were shorter than in wild-type plants (data not shown), 6 which ultimately resulted in dwarf adult plants. 7 8 Supplementary experimental procedures 9 10 Plant material and growth conditions 11 For the identification of homozygous mutant plants obtained from the 12 SALK collection, specific oligo combinations were used to genotype them by 13 PCR analysis: ATHB4 (JO314 + JO285, and JO291 + JO292), athb4-1 (and 14 LBb1 + JO285), athb4-2 (and LBb1 + JO292), HAT1 (CSO23 + CSO24), hat1-1 15 (LBb1 + CSO24), hat1-2 (CSO23 + LBb1), HAT2 (JO340 + JO383, and RO3 + 16 RO4), hat2-1 (LBb1 + JO383), hat2-2 (LBb1 + RO4), hat2-3 (RO3 + LBb1), 17 HAT3 (CSO11 + CSO12) and hat3-1 (LBb1 + CSO12). Homozygous lines were 18 selected for further analysis after outcrossing once to Col-0. 19 20 Generation of constructs to overexpress ATHB4 21 The whole coding sequence of ATHB4 was PCR-amplified with specific 22 primers (JO284 and JO285) using cDNA as a template. The cDNA was 23 prepared from total RNA extracted of Col-0 seedlings treated for 1h with 24 simulated shade using Moloney Murine Leukemia Virus Reverse Transcriptase 2 1 (Invitrogen, www.invitrogen.com), following manufacturer’s protocol. The PCR 2 fragments containing the coding region were cloned into pTZ57T/R (Fermentas, 3 www.fermentas.com). The resulting vector pSP22 was sequenced for identity 4 confirmation. The plasmid was digested with KpnI and BamHI, and cloned in 5 pBinAr (Hofgen and Willmitzer, 1990) digested with the same restriction 6 enzymes to give pBF10 (P35S:ATHB4). 7 ATHB4 sequence was amplified without the stop codon using specific 8 primers (JO284 + CSO7) using cDNA prepared as before as a template. The 9 corresponding PCR fragments were subcloned in pCRII-TOPO (Invitrogen) to 10 give pCS12. The insert was sequenced and no additional mutations were found 11 in the coding region. pCS12 was digested with XbaI and BamHI and cloned in 12 frame with the GR domain in the pGREEN0029 vector (Hellens et al., 2000) 13 digested with the same restriction enzymes to give pCS13 (ATHB4-GR). The 14 EcoRI-HindIII fragment from pBinAr, which contains the 35S promoter and the 15 OCS terminator cassette, was subcloned into the same restriction sites of the 16 binary vector pCAMBIA1300, which confers hygromycin resistance to transgenic 17 plants, to give pCS14. The EcoRI fragment from pCS13 was blunt-ended with 18 Klenow and subcloned into SmaI-digested pCS14, generating pCS19 19 (P35S:ATHB4-GR). 20 21 RNA Blot Analysis 22 Probes for RNA blot analyses were made by amplifying Col-0 genomic 23 DNA with specific primers: CSO9 and CSO10 for HAT1; CSO11 and CSO12 for 24 HAT3; CSO17 and CSO18 for HAT22; RO1 and RO2 for IAA1; and BO42 and 3 1 BO43 for At5g45670. PCR products were subcloned into pCRII-TOPO or 2 pBSK+ to give pCS15, pCS20, pCS17, pIR3 and pJB31 respectively. Inserts 3 were sequenced for identity confirmation. Probes for DNA inserts, isolated by 4 restriction digestion or by PCR using specific primers, were radioactively labeled 5 with [α32P]dCTP by using a random primed DNA-labeling kit (Roche Molecular 6 Biochemicals, www.roche-applied-science.com), and purified through Sephadex 7 G-50 column (Amersham, www.gehealthcare.com). Images were visualized by 8 using a molecular imager FX (Bio-Rad, www.bio-rad.com), and band intensities 9 were quantified by using QUANTITY ONE (Bio-Rad) software. Expression levels 10 were calculated relative to the “wild-type non treated” value of each set of 11 samples after normalization with the 25S rRNA signal. 12 13 Primers used 14 The sequence of the primers used for probe generation and plasmid 15 construction is: BO42 (5’-TAG-AAA-AAG-ATG-GCG-AGA-ATG-AGT-3’), BO43 16 (5’-GAT-CTT-TCT-CTC-TTT-AGA-GAG-ATG-C-3’), CSO7 (5´-GGG-GAT-CCG- 17 CGA-CCT-GAT-TTT-TGC-TG-3’), CSO9 (5’-ATC-ATG-ATG-ATG-GGT-AAA- 18 GAG-GAT-TTG-3´), 19 ATC-3’), 20 CSO12 (5’-ACT-CTA-ATG-AGA-ACC-AGC-AGC-AGG-TCG-3’), CSO17 (5’- 21 CAA-ATG-GGT-CTT-GAT-GAT-TCA-TGC-AAC-3’), CSO18 (5’-TAA-CTA-ACA- 22 TGC-TGC-AGA-AGG-ATT-AG-3’), CSO23 (5’-CCA-CAA-GGA-AAC-AAC-ACA- 23 GAT-CC-3’), CSO24 (5’-ATA-CTC-GCA-ATC-CAC-TTC-GGT-C-3’), JO284 (5’- 24 AGG-ACA-ATG-GGG-GAA-AGA-GAT-GAT-3’), CSO11 CSO10 (5’-AAG-TTA-AGA-CCT-AGG-ACG-CAT-CAC- (5’-AAA-ATG-AGT-GAA-AGA-GAT-GAT-GGA-TTG-3’), 4 JO285 (5’-CCT-TCC-CTA- 1 GCG-ACC-TGA-TTT-TTG-3’), JO314 (5’-GTG-TGG-TTT-CAG-AAC-CGT-AGG- 2 3’), 3 JO292 4 JO340 5 CAC-GTC-TAT-CTT-GCG-AAG-3’), LBb1 (Roig-Villanova et al., 2006), RO1 (5’- 6 GTG-AGA-GAA-TAT-GGA-AGT-CAC-C-3’), 7 TAA-GGC-AGT-AG-3’), RO3 (5’-AAC-ATG-ATG-ATG-ATG-GGC-AAA-GAA-G- 8 3’) and RO4 (5’-AAA-TCA-CGA-TCG-TGG-ACG-CAA-GGC-3’). JO291 (5’-GGA-AGC-TTA-CTC-TAC-CAT-CCA-CTA-ATG-TTT-TC-3’), (5’-GTC-GGA-TCC-ACC-ATT-GTC-CTC-AAC-AGA-AAG-AAC-TT-3’), (5’-GGG-GGA-CAC-AAG-TTG-GAG-ATA-AAG-3’), RO2 JO383 (5’-GTT- (5’-GAC-AAT-GGA-TCA- 9 The primers used for qPCR were BO33 (5’-GCG-GTC-TAT-GTA-GGA- 10 GAG-AAT-GAT-C-3’) and BO34 (5’-CCG-GCA-CCA-CAT-ATC-TCT-TCT-T-3’) 11 for SAUR15, BO35 (5’-AAA-CGC-AAA-GAA-GCT-TAT-GAA-GAT-G-3’) and 12 BO36 (5’-GCT-GCT-CTT-TGT-TGC-CAT-TTC-3’) for SAUR68, BO59 (5'-GCC- 13 TTA-TGA-TCC-ATT-GTC-TCA-AAA-CA-3') and BO60 (5'-TTG-CTT-TTT-CTT- 14 TCT-TTA-CAC-CAA-ACT-3') for IAA1, BO68 (5'-AGA-AGT-GAG-AGA-GAA- 15 GGA-ATC-TCC-G -3') and BO69 (5'-TCA-TCT-GAG-GTT-CCA-CGT-GAG-TA- 16 3') for HAT2, and BO40 (5’-AAA-TCT-CGT-CTC-TGT-TAT-GCT-TAA-GAA-G- 17 3’) and BO41 (5’-TTT-TAC-ATG-AAA-CGA-AAC-ATT-GAA-CTT-3’) for UBQ10 18 (At4g05320). 19 20 Supplementary References 21 Hellens, R., Edwards, E., Leyland, N., Bean, S. and Mullineaux, P. (2000) 22 pGreen: a versatile and flexible binary Ti vector for Agrobacterium- 23 mediated plant transformation. Plant Mol Biol, 42, 819–832. 5 1 Höfgen, R. and Willmitzer, L. (1990) Biochemical and genetic analysis of 2 different patatin isoforms expressed in various organs of potato. Plant Sci, 66, 3 221-230. 6