MALIGNANT MELANOMA
advertisement

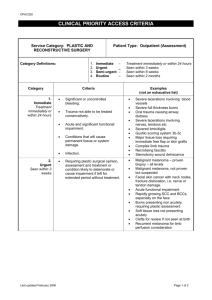
MALIGNANT MELANOMA Staging: AJCC Staging System — The AJCC staging system is based upon evaluations of the primary tumor (T) and the presence or absence of regional lymphatic (N) and distant metastases (M) . The AJCC staging system divides patients into four stages: Stage I — Stage I is limited to patients with low-risk primary melanomas, without evidence of regional or distant metastases. Stage I is divided into stages IA and IB: Stage IA primary lesions are 1 mm thick, without either ulceration of the overlying epithelium or invasion of the reticular dermis or subcutaneous fat (Clark level IV or V) Stage IB includes primary lesions 1 mm thick with epithelial ulceration or invasion into Clark's levels IV or V. Stage IB also includes primary lesions 1 mm and 2 mm thick without ulceration or invasion into Clark's levels IV or V. Stage II — Stage II disease includes high-risk primary tumors without evidence of lymphatic disease or distant metastases. Stage II is divided into three subsets: Stage IIA includes lesions 1 mm and 2 mm thick with ulceration of the overlying epithelium and those >2 mm and 4 mm thick without epithelial ulceration. Stage IIB lesions are >2 mm and 4 mm thick with epithelial ulceration or >4 mm without ulceration. Stage IIC consists of primary lesions >4 mm with epithelial ulceration. Stage III — Pathologically documented involvement of regional lymph nodes or the presence of in transit or satellite metastases defines a lesion as stage III. Within stage III, patients with involvement of 1, 2 to 3, and 4 or more lymph nodes are sub-classified as having N1, N2, and N3 disease, respectively. Patients with in transit or satellite metastases are classified as having N2 disease if lymph node involvement is not present, and as having N3 disease if lymph node involvement is present. In addition, lymph node involvement is divided based upon whether disease is microscopic or macroscopic (eg, N1a versus N1b). Among patients with stage III disease, the presence of ulceration in the primary tumor remains an independent negative prognostic factor. Using these parameters, patients with stage III disease are sub-classified as follows: Stage IIIA includes patients with one to three microscopically involved lymph nodes (N1a or N2a) in a patient whose primary tumor is not ulcerated. Malignant Melanoma Management Guidelines Page 1 of 8 Stage IIIB includes patients with one to three microscopically involved lymph nodes (N1a or N2a) in a patient whose primary tumor is ulcerated, or one to three macroscopically involved lymph nodes (N1b or N2b) in a patient with a nonulcerated primary tumor, or patients with in transit and/or satellites without metastatic lymph nodes (N2c). Stage IIIC is defined by the involvement of four or more lymph nodes, matted lymph nodes, or the presence of in transit metastases or satellite lesions in conjunction with lymph node involvement (ie, N3), or patients with one to three macroscopically involved lymph nodes (N2b) in a patient whose primary tumor is ulcerated. Patients with clinical evidence of regional lymph node without a full regional lymph node dissection are classified as clinical stage III, and no further staging is applied. Stage IV — The presence of distant metastases defines stage IV disease. Patients with metastases are divided into three categories: disease limited to distant skin, subcutaneous tissues, or lymph nodes (M1a), lung metastases (M1b), or involvement of all other visceral sites (M1c). In addition, the presence of an elevated serum LDH in conjunction with any distant metastasis classifies a patient as having M1c disease. Malignant Melanoma Management Guidelines Page 2 of 8 AJCC 2002 TNM classification for melanoma Tumor (T) classification TX Primary tumor cannot be assessed (eg, shave biopsy, regressed primary) Tis Melanoma in situ T1 < or = 1.0 mm A: without ulceration and level II/III* B: with ulceration or level IV or V* T2 1.01-2.0 mm a: without ulceration b: with ulceration T3 2.01-4.0 mm a: without ulceration b: with ulceration T4 >4.0 mm a: without ulceration b: with ulceration Node (N) classification N1 One lymph node a: micrometastases (clinically occult) b: macrometastases (clinically apparent) N2 2-3 lymph nodes a: micrometastases b: macrometastases c: in-transit met(s)/satellite(s) without metastatic lymph nodes N3 4 or more metastatic lymph nodes, or matted lymph nodes, or in-transit met(s)/satellite(s) with metastatic lymph node(s) Metastasis (M) classification M1a Distant skin, subcutaneous, or lymph node metastases, normal LDH M1b Lung metastases, normal LDH M1c All other visceral metastases, normal LDH Any distant metastases, elevated LDH * Clark's levels: level II: invades the papillary dermis; level III: invades to the papillary- Malignant Melanoma Management Guidelines Page 3 of 8 reticular dermal interface; level IV: invades the reticular dermis; level V: invades subcutaneous tissue. Micrometastases are diagnosed after elective or sentinel lymphadenectomy. Macrometastases are defined as clinically detectable lymph node metastases confirmed by therapeutic lymphadenectomy or when any lymph node metastasis exhibits gross extracapsular extension. Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original source for this material is the AJCC Cancer Staging Manual, Sixth Edition (2002) published by Springer-Verlag New York, Inc. Stage AJCC 2002 stage groupings for cutaneous melanoma Clinical stage grouping* Pathologic stage grouping 0 Tis N0 M0 pTis N0 M0 IA T1a N0 M0 pT1a N0 M0 IB T1b N0 M0 pT1b N0 M0 T2a N0 M0 pT2a N0 M0 T2b N0 M0 pT2b N0 M0 T3a N0 M0 pT3a N0 M0 T3b N0 M0 pT3b N0 M0 T4a N0 M0 pT4a N0 M0 IIC T4b N0 M0 pT4b NO M0 III Any T N1-3 M0 pT1-4a N1a M0 pT1-4a N2a M0 pT1-4b N1a M0 pT1-4b N2a M0 pT1-4a N1b M0 pT1-4a N2b M0 pT14a/b N2c M0 IIA IIB IIIA IIIB Malignant Melanoma Management Guidelines Page 4 of 8 IIIC IV Any T Any N Any M1 pT1-4b N1b M0 pT1-4b N2b M0 Any T N3 M0 Any T Any N Any M1 * Clinical staging includes microstaging of the primary melanoma and clinical/radiologic evaluation for metastases. By convention, it should be used after complete excision of the primary melanoma with clinical assessment for regional and distant metastases. Pathologic staging includes microstaging of the primary melanoma and pathologic information about the regional lymph nodes after partial or complete lymphadenectomy. Pathologic Stage 0 or Stage IA patients are the exception; they do not need pathological evaluation of their lymph nodes. There are no Stage III subgroups for clinical staging. Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original source for this material is the AJCC Cancer Staging Manual, Sixth Edition (2002) published by Springer-Verlag New York, Inc. Staging Work-up: 1. 2. Stage I-II A Stage II B – III-IV Baseline: CBCD, SP, LDH, CXR CBCD, SP, LDH Chest X-ray CT abdomen CT or MRI head and Neck: with head and neck melanoma CT pelvis: with melanoma below the waistline CT brain or bone scan only if patient is symptomatic CT chest, if chest X-ray is abnormal Biopsy: Full thickness into the subcutaneous tissue must be performed. Shave or curette biopsies are absolutely contra-indicated, since the tumor thickness cannot be ascertained. Types:1. Excisional biopsies- For small tumor <1.5cm, included narrow margins 2mm. The biopsy incision should be oriented so that it can be re-excised with Malignant Melanoma Management Guidelines Page 5 of 8 optimal skin margins and minimal skin loss if the lesions prove to be malignant. 2. Incisional biopsies- For large lesions or when the amount of skin removed is critical, e.g. face, hand or feet. It should not be taken at the periphery of the lesion. Treatment: Summary and Recommendations — The definitive treatment for primary cutaneous melanoma is wide local excision. The available data, taken in aggregate, allow general recommendations to be made regarding the optimal extent of resection based upon the histologic depth of the melanoma. A margin of 0.5 cm of normal skin is recommended for in situ melanomas A 1 cm margin is recommended for melanomas <1 mm thick A 1 cm margin is appropriate for melanomas 1-2 mm thick. Wider margins have not been proven to result in lower local recurrence or mortality. For thicker melanomas, appropriate trials do not exist showing the benefit of margins widths greater than 2 cm; therefore, a 2 cm margin is recommended for melanomas 2 mm thick. Others recommend a surgical margin of 3 cm for T3 (2.1 to 4.0 mm) or T4 (>4 mm) primary tumors, based upon the findings of the British trial discussed above Recommendation for ELND: Value of ELND remains unproven, as randomized trials have not demonstrated a statistically significant improvement in overall survival. There may be subgroups of patients who benefit from ELND, but consensus is lacking on this issue. The availability of lymphatic mapping with SLNB has obviated a possible role for ELND. Stage III melanoma (node positive or intransit metastases) a. WLE 2-3 cm margin with skin (grafting, if necessary) b. Regional lymph node dissection of clinically involved lymph node Recommendation for adjuvant IFN Alpha-2b: Not to be given outside clinical trial, until further clear data is available. Recommendation for ILP (Isolated limb perfusion) Not to be done outside clinical trial Stage IV: a. Multiple metastases: Palliative care and symptomatic therapy c. Solitary metastases, e.g. regional lymph node, lung, GI, or brain may be palliated by resection in selected patients. Malignant Melanoma Management Guidelines Page 6 of 8 Recurrent Melanoma: 1. Local or intransit recurrent confined to extremity or treated with resection for clear margins. 2. Distant cutaneous or soft tissue or parenchymal recurrence can be resected, if isolated lesions. Positive margins can be radiated. Variants 1. Ocular Melanoma: Diagnosis clinical – no biopsies are performed Ultrasound and MRI of the eye are useful in determining extent Treatment:a) For asymptomatic patients with small tumors (<10 mm in diameter and <2 mm in height), we recommend observation, rather than active intervention, until evidence of growth is documented For patients with larger tumors and those with symptoms, we recommend active treatment with either radiation therapy (RT) or enucleation b) For patients who present with metastatic disease or who develop metastatic disease after treatment of their primary tumor, the prognosis is poor. Therapy is generally palliative and experimental 2. Conjunctival melanoma For patients with melanoma arising in the conjunctiva, initial management focuses upon local control of disease. For most patients this includes wide local excision, supplemented by cryotherapy and alcohol application. Malignant Melanoma Management Guidelines Page 7 of 8 Reference: 1. Cosimi AB, Sohar AJ, Mihm MC. Conservative surgical management of superficially invasive cutaneous melanoma. Cancer 1984; 53:1256. 2. Karakonsis C, Balch, Uricht M. Local recurrence in malignant melanoma. Long term result of surgical trial (Abstract) 48th Annual Conference Symposium, 1995. 3. Buzaid, Tinocol, Ross M, et al: The role of computed tomography in the staging of patients with local-regional metastases of melanoma. J Clin Oncol 1995; 13: 2104- 2108. 4. Varonesi U, Cascinello N: Narrow excision (ICM margin): A safe procedure for thin cutaneous melanoma. Archives of Surgery 1991; 126 (4): 438-441. 5. Balch C, Soong S, Basolucci A, et al: Efficacy of an elective regional lymph node dissection of 1-4 mm thick melanoma for patients 60 years or younger. Ann Surg 1996; 224: 255-263. 6. Warnom IL, Soong SJH, Krist MM, et al: Surgery as palliative treatment for distant metastases of melanoma. Annals of Surgery 1986; 204 (2): 181-185. 7. Overette TK, Stiu MH: Surgical treatment of distant metastatic melanoma: Medications and results of cancer. 1985; 56(5): 1222-1230. 8. Manshof W, Van Peperzeel H: Choroidal melanoma: Enucleation and observation? A new approach. Arch Ophthalmol 1980; 98: 71-77. 9. Creagan E, Dalton R, Ahmann D, et al: Randomized surgical adjuvant clinical trial of R-EFN Alpha 2A. JCO 1995; 13: 2776-2778. 10. Ringburg U, Andersson R Eldh J: Resection margins of 2 versus 5 cm, for cutaneous malignant melanoma with a tumor thickness of 0.8 – 2.0 mm. Cancer 11. 1996; 77(9): 1809-1814. 12. Lienard D. Regional therapy in melanoma. 23rd ESMO Congress November 1998. Educational book 35-36. Malignant Melanoma Management Guidelines Page 8 of 8