Signal Transduction in Neurobiology 19 November 2007
advertisement

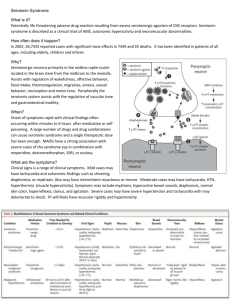
Mechanistic and Genetic Causes of Depression and the Effect of SSRIs on the Serotonergic Pathway Ashley Nicole Phares Introduction Depression is a very common disorder and up to 20% of the population in the United States suffers from this condition, with twice as many females diagnosed as males (15,16). Studies have shown that 40-50% of depression risk is genetic, while the other portion consists of non-genetic factors like viral infection, emotional trauma, and stress (16). Diagnosis requires the suffering of a major depressive episode marked by several specific symptoms, including depressed mood and loss of interest (14). The hippocampus is generally considered the prominent site for the generation and treatment of depression, though abnormalities related to the disorder also extended far beyond this region (16). These include two major serotonergic pathways in the brain that are located in the projections ascending from the medial and dorsal raphe and the descending projections from the caudal raphe into the spinal cord (18). Research has established a pathophysiological role of serotonin, also known as 5-hydoxytryptamine (5-HT), in depression (22, 8). In humans, serotonin expressing neurons are highly localized to the spinal cord and brainstem in clusters. In these collections, neurons send out axons whose terminals contain serotonin and innervate many different areas throughout the brain (22). If part of the serotonergic pathway is disrupted, the signaling system will not operate correctly and will therefore likely lead to depression. But how might this pathway be disrupted? As mentioned previously, genetics play a large role in determining risk for depression (16). If mutations occur in genes coding for elements of the pathway, there is a stronger likelihood for depressive tendencies (16, 3). Yet while genes predispose many people to depression, others may be affected by epigenetic mechanisms (21). For instance, longterm alterations associated with depression are also thought to stem from adverse experiences early in life (14, 10). Early environmental stressors result in an enhanced biologic stress mechanism (21). Synergistically, stress and genetic risk initiate a cascade that disrupts the dynamic serotonergic system through neurobiological transformations. Other factors may include high levels of the stress hormone cortisol, or imbalanced densities of glucocorticoid and/or mineral corticoid receptors (14). In fact, increased levels of glucocorticoid downregulate hippocampus 5-HT, and many patients diagnosed with depression exhibit enlarged adrenal glands. Although it is difficult to reduce the many emotional and physical symptoms of depression to one prominent cause, the most widely acknowledged source of this condition is indeed an imbalance of the neurotransmitter, serotonin. Solid evidence has shown that low serotonergic activity is directly linked to major depression disorder (10). The Mechanics of the Serotonergic Pathway (Figure A) We know that, when altered, the serotonergic pathway can cause depression, but what is its normal function and how does it work? Typically, 5-HT governs autonomic operations, influences learning and memory, and affects behavior and mood (12). When serotonin is released by the pre-synaptic cell into the synaptic cleft, it binds to one of its distinct subtypes of 5-HT receptors, resulting in a signal transduction cascade. The serotonergic neurotransmission is terminated when 5-HT is rapidly re-uptaken into the presynaptic terminus. The re-uptake is controlled by the serotonin transporter, or SERT (12). [See Figure A] The brain synthesizes serotonin from the amino acid L-tryptophan. This amino acid comes mainly from the diet and must travel across the blood-brain barrier by active transport in order to reach the brain (18). L-tryptophan metabolism is a highly regulated physiological process (19). Tryptophan hydroxylase (TPH) is the rate-limiting enzyme in the biosynthetic pathway for serotonin. Its role is to convert the tryptophan amino acid into 5-hydroxytryptophan (5-HTP) which in turn is decarboxylated to 5-HT (3). [See Figure B] Levels of serotonin are linked to levels of L-tryptophan, which change with both diet and levels of competing amino acids (18). Pre-cursor loading and increased intake of tryptophan can increase serotonin concentrations (9). Tryptophan depletion leads to decreased levels of 5-HT and often depression (18). (Figure B) Serotonin receptors are the targets for the 5HT neurotransmitter and are primarily located on the surface of the post-synaptic terminal (11). Research has established that there are at least seven distinct receptor families, each with multiple subtypes (18). These include 5-HT1A, 5-HT1D, 5HT3, and most importantly, 5-HT2A, 5-HT2B, and 5-HT2C which each affect a distinct neural system (22, 13). Direct-acting agonists and antagonists often show selective affinity for a specific family and subtype of receptor (9). Perhaps the most studied subtype of receptors is the 5-HT2C (11). This subtype is widely distributed throughout the brain and is considered a member of the class A Gprotein-coupled receptor homodimers which act in intracellular signaling pathways (11, 20). It couples to G alpha q, phospholipase D, and arachidonic acid metabolism. 5-HT2C receptors function as homodimers, regardless as to whether they are in the active of inactive conformation. These two conformations result from receptor isoforms produced by RNA editing (11). 5-HT also acts on some pre-synaptic receptors, such as 5-HT1B/1D and 5-HT1A, which act in a negative feedback mechanism. When bound by a neurotransmitter, they inhibit firing rates, therefore decreasing the amount of serotonin released into the synaptic cleft (18). The serotonin transporter mediates the reuptake of the neurotransmitter from the synaptic cleft, back to the pre-synaptic nerve terminal (12). It is found on serotonin neuron processes, nerve terminals, and also platelets and its density varies according to location (3). The SERT is an integral membrane protein that belongs to the Na+/Cldependant family that transports metabolites and neurotransmitters, as well as moves monoamines and amino acids through the lipid bi-layer (12). The human serotonin transporter is a twelve membranespanning protein that is affectively a neuronal uptake pump for 5-HT (22). Many different mechanisms, such as protein kinase C triggered internalization, control SERT activity. It has been found that with prolonged administration of serotonin re-uptake inhibitors, adaptive changes in SERT mRNA and protein levels occur (12). Each of these parts of the serotonergic pathway are integral to the working system and if altered, will likely result in an increased risk for depression. Genetic Mutations to the Serotonergic Pathway Components Evidence of genetic causation comes from observation of a link between depression and family history, twin comparisons, and twin adoption studies (3). Also, as stated before, epidemiological studies have shown that 40-50% of depression risk is derived from genetic variation (16). Since each of the components of the 5-HT pathway are so essential for correct function, if any mutations occur in genes coding for a part of the serotonergic system, problems are bound to occur (3). Thus, genes related to this system are prime targets for investigation in the search for the causes of depression (3, 15). Polymorphisms in these genes can contribute to altered forms, or isoforms, that display different levels of wildtype functionality (3). Worthy of study, are the polymorphisms of the serotonin biosynthetic enzyme, tryptophan hydroxylase, or TPH. Since alteration in 5-HT neurotransmission is implicated in depression, it makes sense to look at this important member of serotonin’s synthetic pathway. The TPH gene is located on chromosome 11 and two polymorphisms in intron seven have been detected, which are in a tight linkage disequilibrium. These consist of A to C substitutions in either nucleotide 779 or 218. Less TPH is not apparent in the dorsal raphe nucleus of depressed suicide victims, which predicts that this enzyme protein may be altered in the isoforms so as to have less activity (3). Altered levels of 5-HT receptors are often reported in depression (1). For example, a reduced number of serotonin receptors has been observed in the post-mortem hippocampus of depressed suicides (10). This eludes that genes coding for the 5-HT receptors are also prime targets for study (2, 3). Two polymorphisms for the 5-HT1B receptor have been detected at nucleotides 129 and 161. These involve silent mutations of a C to a T and a G to a C respectively. These polymorphisms were found at similar frequencies in normal and depressed patients, but exhibit a lower level of binding in the latter. Studies have also detected an uncommon substitution of a phenylalanine residue for a cysteine (3). Polymorphisms have additionally been found in genes coding for 5-HT2C receptors. Recent studies have shown that life stress increases production of 5-HT2C receptor isoforms, which have reduced 5-HT sensitivity. Moreover, genetics and life stress early on are synergistic in producing more of these receptor isoforms (5). Two prominent polymorphisms have been seen in the 5-HT2A receptor gene. In one, a T is changed to a C at nucleotide 102 and in the other, an A is replaced by a G at 1438. Other less frequent polymorphisms of 5-HT2A were observed as well (3). However, strong evidence showing that alterations in this subtype result in increased risk for depression have not been shown (15). The human gene for the serotonin transporter is located on chromosome 17. It has been found that less 5-HTT expression and binding may result from a 44 bp insertion/deletion in the 5’ regulatory region adjacent to the promoter. It appears that this polymorphism results in a decreased number of binding sites (3). There is mounting evidence that single nucleotide and simple repeat sequence polymorphisms in coding and regulatory genes of the serotonergic pathway can result in a significant difference in the functionality of the system (7). Mutations associated with the genes coding for components of the 5-HT pathway, no matter how small, are certainly worthy of investigation for the role they likely play in the increased risk for depression. The Effect of SSRIs on the Serotonergic Pathway Much research has been done in order to find mechanisms to treat the causes and symptoms of depression. One of the major solutions to this search has been the discovery and implementation of selective serotonin reuptake inhibitors (SSRIs). SSRIs were developed to target and inhibit the serotonin transporter, or neuronal uptake pump. This mechanism involves altering the 5-HT system in order to increase the level of neurotransmitter in the synaptic cleft (22, 23). [See Figure C] Inhibition of the transporter yields an increase of serotonin in the synapse, and therefore an increased activation of the presynaptic inhibitory receptors. Over the period of about three weeks, the negative feedback mechanism achieves a therapeutic effect (18). Imipramine was the first compound shown to block the reuptake of serotonin by the transporters and since then citalopram, fluvoxamine, fluoxetine, paroxetine, sertraline, duloxetine, and others have been added to that list (22, 7, 17). SSRIs have common serotonin agonists that meditate their effects. As far as affecting the uptake pump, SSRIs have proven to be selective. They affect particular receptors that in turn affect a multitude of particular neural systems; hence, no SSRI works exactly the same (22). Selective serotonin reuptake inhibitors vary in elimination half-life, selectivity for serotonin ratios, and affinity for the 5-HT receptor (18). In order to be effective, regular levels of serotonin must still be available. This was shown by administering depletion of tryptophan, the precursor of serotonin, and observing the effects of the SSRIs. Without adequate serotonin being produced, the SSRIs had no positive effect (4, 18). In fact, serotonin depletion leads to reoccurrence of symptoms in up to 80% of patients (22). depression that are just waiting to be discovered and refined. (Figure D) Conclusion (Figure C) With continuous exposure to SSRIs, postsynaptic receptors in the brain often become desensitized. It is hypothesized that this may specifically focus on the 5-HT1A autoreceptors in the midbrain raphe nucleus, increasing serotonin in critical brain regions (22). Application of monoamine reuptake inhibitors leads to an increase in cAMP (cyclic adenosine 3-5 monophosphatase) activation, which turns on protein kinase A, which in turn regulates target genes. This leads to an increase in BDNF synthesis (brain-derived neurotrophic factor) which is a primary neurotrophin of the hippocampus which promotes cellular resilience and long-term potentiation (14). [See Figure D] SSRIs bring relief for many patients suffering from depression by altering an important step in the serotonergic pathway. Possibilities still remain for different, and possibly more effective, ways of treating the causes and symptoms of Depression is a common disorder that plagues a large percentage of the population. One of the main causes of this condition has been linked to alterations in the serotonergic pathway for neurotransmission. Negative outcomes often occur when mechanistic and genetic changes produce a change in the 5-HT system, resulting in an increased risk for depression. Through increased understanding of the serotonin pathway and the genetics governing its components and function, treatments such as SSRIs, can and will continue to be developed and perfected in order to treat the causes and symptoms of depression. References 1 Albert, P.R., Lemonde, S. (2004) 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 10(6): 575-93. 2 Anguelova, M., Benkelfat, C., Turecki, G. (2003) A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: I. Affective disorders. Mol Psychiatry. 8(6): 574-91. 3 Arango, V., Huang, Y., Underwood, M.D., Mann, J.J. (2003) Genetics of the serotonergic system in suicidal behavior. Journal of Psychiatric Research. 37: 375386. 4 Argyropoulos, S.V., Hood, S.D., Adrover, M., Bell, C.J., Rich, A.S., Nash, J.R., Rich, N.C., Witchel, H.J., Nutt, D.J. (2004) Tryptophan depletion reverses the therapeutic effect of selective serotonin reuptake inhibitors in social anxiety disorder. Biol Psychiatry. 56(7): 503-9. 5 Bhansali, P., Dunning, J., Singer, S.E., David, L., Schmauss, C. (2007) Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the alpha subunit of the heterotrimeric G-protien Gq. Journal of Neuroscience. 27(6): 1467-1473. 6 Dell’Osso, B., Allen, A., Hollander, E. (2005) Fluvoxamine: a selective serotonin re-uptake inhibitor for the treatment of obsessive compulsive disorder. Expert Opin Pharmacother. 6(15): 2727-40. 7 D’Souza, U.M., Craig, I.W. (2006) Functional polymorphisms in dopamine and serotonin pathway genes. Hum Mutat. 27(1): 1-13. 8 Freeman, S.L., Glatzle, J., Robin, C.S., Valdellon, M., Sternini, C., Sharp, J.W., Raybould, H.E. (2006) Ligand-induced 5-HT3 receptor internalization in enteric neurons in rat ileum. Gastroenterology.131(1): 97-107. 9 Fuller, R.W. (1991) Role of serotonin in therapy of depression and related disorders. J Clin Psychiatry. 52 Suppl: 52-7. 10 Graeff, F.G., Guimaraes, F.S., De Andrade, T.G.C.S., Deakin, J.F.W. (1996) Role of 5-HT in stress, anxiety, and depression. Pharmacology Biochemistry and Behavior 54(1): 129-141. 11 Herrick-Davis, K., Grinde, E., Weaver, B.A. (2006) Serotonin 5-HT2C receptor homodimerization is not regulated by agonist or inverse agonist treatment. European Journal of Pharmacology. 568: 45-53. 12 Jess, U., El Far, O., Kirsch, J., Betz, H. (2002) Interaction of the C-terminal region of the rat serotonin transporter with MacMARCKS modulates 5-HT uptake regulation by protein kinase C. Biochemical and Biophysical Research Communications. 294: 272-279. 13 Leysen, J.E. (2004) 5-HT2 receptors. Curr Drug Targets CNS Neurol Disord. 3(1): 11-26. 14 Maletic, V., Robinson, M., Oakes, T., Iyenger, S., Ball, S.G., Russell, J. (2007) Neurobiology of depression: an integrated view of key findings. Int J Clin Prac. 61(12): 2030-40. 15 Minov, C., Baghai, T.C., Schule, C., Zwanger, P., Schwartz, M.J., Zill, P., Rupprecht, R., Bondy, B. (2001) Serotonin-2A-receptor and –transporter polymorphisms: lack of association in patients with major depression. Neuroscience. Letters. 303: 119122. 16 Nestler, E.J., Barrot, M., DiLeone, R.J., Eisch, A.J., Gold, S.J., Monteggia, L.M. (2002) Neurobiology of depression. Neuron. Vol. 34: 13-25. 17 Norman, T.R. (2005) Prospects for the treatment of depression. Aust N Z J Psychiatry. 40(5): 394-401. 18 Nutt, D.J., Forshall, S., Bell, C., Rich, A., Sandford, J., Nash, J., Argyropoulos, S. (1999) Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. Eur. NeuroPsychopharmacology. 9 Suppl. 3: S81-S86. 19 Ruddick, J.P., Evans, A.K., Nutt., D.J., Lightman, S.L., Rook, G.A., Lowry, C.A. (2006) Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med. 8(20): 1-27. 20 Serretti, A., Artioli, P., De Ronchi, D. (2004) The 5-HT2C receptor as a target for mood disorders. Expert Opin Ther Targets. 8(1):15-23. 21 Shelton, R.C. (2007) The molecular neurobiology of depression. Psychiatr Clin North Am. 30(1): 1-11. 22 Vaswani, M., Linda, F.K., Ramesh, S. (2003) Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Progress in Neuro- Psychopharmacology & Biological Psychiatry. 27: 85-102. 23 Weizman, A., Weizman, R. (2000) Serotonin transporter polymorphism and response to SSRIs in major depression and relevance to anxiety disorders and substance abuse. Pharmacogenomics. 1(3): 335-41. Ashley is a junior double majoring in biology and classics with a minor in the Blount Undergraduate Initiative. She has researched for three years in the Caldwell genetics lab using the C. elegans model organism. During her freshman year, she worked on the genetic causes of Parkinson’s disease, but now focuses on the study of cancer mechanisms. After graduation, Ashley will pursue a career in dentistry.