Werner Mormann Mohamed Al
advertisement

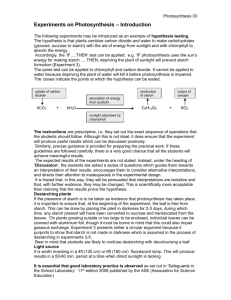
Werner Mormann Mohamed Al-Higari Universitat Siegen, FB 8, Laboratorium fur Makromolekulare Chemie, Siegen, Germany Acylation of Starch with Vinyl Acetate in Water Acetylation of different types of starch with vinyl acetate in water in the presence of a base is described. Maximum degree of substitution under these conditions is 1.1. Vinyl esters of higher homologous acids (vinyl laurate) react very slowly giving very low degrees of substitution after long reaction times. The reaction mixture is biphasic, because vinyl esters are not miscible with water. Formation of two layers reduces loss of acylating agent due to reaction with water - which is a major problem encountered for esterification with acid anhydrides or chlorides - to less than 5%. Unlike acylation of starch in DMSO with vinyl esters, the acetates of starch obtained by the method reported do not show regioselective distribution of acetyl groups within the anhydro-glucose units. Keywords: Acetylation; Vinyl acetate; Base catalysis; Substituent distribution 1 Introduction Esterification of starch to low degrees of substitution (DS) is mostly made to reduce the gelatinization temperature or to improve solution stability by preventing retrograda-tion. Higher degrees of substitution are necessary to achieve solubility in organic media. The α-D-glycosidic bond between anhydroglucose units (AGU) in starch is very sensitive to acid catalyzed hydrolysis, which causes degradation of the polymeric chain of starch. Because of the sensitivity to acid hydrolysis esterification is usually made in the presence of a base such as sodium hydroxide [1] or pyridine [2] using acid anhydrides or acid chlorides as acylating agents. These methods, however, have not gained technical importance. Acylation of starch with vinyl esters is an alternative approach that does not require molar amounts of base as an acid scavenger. This method has been investigated by several research groups to synthesize starch acetates with low DS. Acylation with vinyl acetate in aqueous medium was studied by Tuschhoff [3], Joosten [4] and Mezynski [5] and reported mainly in patents. The degrees of esterification obtained by these authors were between 0.1 and 0.35. Klemm and co-workers studied acylation in organic solvents like dimethyl sulfoxide with enzymatic or base catalysis [6]. They established conditions for regioselective acylation of the 2-hydroxy group [7], [8]. Degrees of esterification in the order of 1 or higher have not been obtained in aqueous media. Synthesis of highly esterified materials, especially if obtained with equivalent Correspondence: Werner Mormann, Universitat Siegen, FB 8, Laboratorium fur Makromolekulare Chemie, Adolf-Reichwein-Straße 2, 57068 Siegen, Germany. Phone: +49-271-740-4713, Fax: +490271-740-2226, e-mail: mormann@chemie.uni-sie-gen.de. amounts or only slight excess of acylating agent, could be a significant progress in the manufacture and availability of starch ester of organic acids. In the present paper we describe an improved method for the synthesis of starch acetates by acylation with vinyl acetate in water. 2 Materials and Methods 2.1 Materials Potato starch, corn starch, wheat starch (Fluka, Buchs, Switzerland), white potato starch dextrin Avedex W80, yellow potato starch dextrin Avedex LC36 (Avebe, Foxhol, The Netherlands) were used as received. 2.2 Methods of characterization IR-spectra were recorded on a Bruker Equinox 55 FTIR spectrometer as films on NaCI plates; 1Hand 13C-NMR spectra (Bruker AC-200 or WH-400 FT, Bruker, Karlsruhe, Germany) were obtained in deuterochloroform or DMSO-d6 using the non-deuterated compounds as internal standard. Thermal properties were determined on a Mett-ler TA 4000 System with DSC 30 and TG 50 equipment. 2.3 Acylation of starch: general procedure A 500 mL flange flask with internal thermometer, mechanical stirrer and reflux condenser with bubble counter was charged with 200 g (1.23 mol AGU) potato starch, 15.76 g (9 mol% with respect to AGU) potassium carbonate in 100 mL water and 212.4 g (2.46 mol) vinyl acetate. The mixture was stirred under gentle reflux while acetalde- hyde was formed. After the reaction had finished, unreacted vinyl ester was distilled off, the emulsion-like product was precipitated in isopropanol, isolated by filtration and dried at 60°C/20 Pa. Amounts, catalysts, reaction conditions, yields, and degree of esterification are given in the respective tables. 2.4 Complete acetylation of partially acylated starch with acetic anhydride-d6[9] Potato starch acetate (147 mg, 0.74 mmol) (DS 1.1) and 660 mg (6.1 mmol) acetic anhydride-d6 in 12.8 mL pyri-dine were stirred at 60°C until signals of hydroxy groups were no longer visible in the IR-spectrum (48 h). Half of the solvent was distilled off and the starch was precipitated in 100 mL methanol. The product was centrifuged and the supernatant liquid was removed, this procedure was repeated twice, then the solid was filtered, dissolved in benzene and isolated by freeze drying and drying at 20 Pa. 3 Results and Discussion Acylation with vinyl esters proceeds via an addition-elimination mechanism with formation of ester groups and acetaldehyde, which is gaseous under the reaction conditions and thus can be used to follow the progress of the reaction. The reaction is represented in Equation 1. Reactions were carried out with vinyl acetate and vinyl laurate as acylating agents. Acylation with vinyl esters requires base catalysis. Potassium carbonate and diso-dium hydrogenphosphate were used for this purpose; triethylamine as an organic base was also included in the present study. In a series of experiments reaction conditions were varied to determine optimum conditions for acylation of starch. Variables were the concentration of starch in water, amount and type of catalyst, reaction time and temperature. Criterion of success was the degree of esterification (degree of substitution: DS) achieved. The DS was determined by 1HNMR-spectros-copy. Details of experimental conditions and results are given in Tab. 1. Reaction temperatures studied were 80 and 100°C. The latter gave better results and was used in further experiments. The concentration of starch in water obviously plays an important role. A 20% concentration resulted in a DS 0.67 after 50 h, 30% concentration gave a DS of 1.0 after 24 h. Further reduction of water, i.e. concentration of starch of 66% and reduction of the amount of vinyl acetate with respect to AGU from 1 :4 to 1:2 gave a DS of 1 already after 5 h without significant further increase Tab. 1. Acylation of starch in water (100°C bath temperature). after 24 h reaction time. Disodium hydrogenphosphate as catalyst is less efficient than potassium carbonate, which has been reported to be a good catalyst in DMSO [7]. Use of triethylamine as cosolvent rather than as catalyst was the only experiment that gave a DS significantly higher (1.4) than obtained in completely aqueous medium. The type (provenience) of starch is not crucial for the reaction. Using the optimum reaction conditions established for potato starch, corn starch, wheat starch and two potato starch dextrins could be acetylated to a DS of approximately 1, with white potato starch dextrin even 1.26 was achieved. In an attempt to investigate the applicability of this method to other acids, vinyl laurate (dodecanoate) was used under otherwise identical conditions. In this case a DS of only 0.2 was achieved after 48 h. This may be attributed to the low solubility of vinyl laurate in water and of starch in vinyl laurate. To find out whether the limit of degree of esterification close to 1.0 is inherent to starch or caused by the fact that some kind of heterogeneity is the limiting factor DMSO was used as reaction medium. Dicke [7] reported regioselective acylation of the hydroxy group in the 2-position of starch working in DMSO as solvent for starch when the reaction was carried out at 40-70°C with 2% disodium hydrogenphosphate as catalyst. The results in Tab. 2 show that a DS of 1.6 could be obtained if the temperature was increased to 110°C. In this series a 50% solution of starch gave a lower DS (0.75) than a 10% solution. With vinyl laurate a DS of 2.3 was obtained under these conditions. Acylation of starch with anhydrides or acid chlorides is accomplished with high excess of acylating agents, especially if carried out in water containing reaction media. To study occurrence and extent of hydrolysis of vinyl acetate, the amounts of liquid components after reaction were determined by gas chromatography in one experiment. Liquids were completely removed under normal pressure, then in vacuum and collected in a flask cooled with liquid nitrogen to avoid losses of volatiles. Two phases formed; tetradecane was used as internal standard for the organic phase and diethyleneglycol mono-methylether for the aqueous phase. Amounts before and after reaction are given in Equation 2. The organic phase contained 0.86 mol vinyl acetate and no acetic acid, while 9 mmol of acetic acid were found in the aqueous phase, which means that less than 5% of the acylating agent was lost by hydrolysis. By consequence and within experimental error acylation takes place with stoichiometric amounts of vinyl acetate and the excess used can be recovered. These favorable conditions for acylation are most likely due to the immis-cibility of vinyl esters and water. A minimum of solubility, on the other hand, is required to enable reaction with the hydroxy groups of starch. This explains why acylation with vinyl dodecanoate is too slow to be considered as an alternative to acylation in organic solvents (cf. Tabs. 1 and 2). Amylose containing starch types with DS from 0.9 to 1.2 are soluble (10%) in water and dipolar aprotic solvents like DMSO, dimethyl formamide and dimethyl acetamide and insoluble in alcohols, ketones, ethers, and aromatic hydrocarbons. 6-position [7], approximately 46% of C2-hydroxy groups, 29% of C3- and 28% of C6-hydroxy groups have been esterified. This does not correspond to the expected reactivity of hydroxy groups (2 > 6 > 3) but is rather a random distribution with some preference for the 2-position. 3.1 Regioselectivity of acetylation of potato starch As acetylation of starch in DMSO under the conditions reported by Dicke [7] gave 2-O-acetyl starch, we investigated the distribution of acetyl groups within the AGUs. Partially acetylated starch contains non-, mono-, di-, and tri-substituted AGUs. NMR-methods were used to determine amount and distribution of acetyl groups within the AGUs [9]. For better resolution of signals residual hydroxy groups were completely acetylated with deuterated acetic anhydride (cf. Eq. 3). This imparts solubility of the triacetates in deuterated chloroform which is the solvent of choice [9], and allows to assign the signals of non-deut-erated acetyl groups to the 2-, 3-, and 6-positions of the AGU. In Fig. 1 the proton NMR-spectrum of acetylated potato starch (DS 1.1, entry 5b Tab. 1) is shown. The resonances of the acetyl protons in the different positions of the AGU cannot be base-line separated in a 400 MHz spectrum. It can be seen, however, that there is no regiospecificity of acetylation, because significant signals for all three positions are present. Neglecting the signal at 2.01 ppm, which is not caused by acetyl groups in the 2-, 3-, or 6-position [7], approximately 46% of C2-hydroxy groups, 29% of C3- and 28% of C6-hydroxy groups have been esterified. This does not correspond to the expected reactivity of hydroxy groups (2 > 6 > 3) but is rather a random distribution with some preference for the 2-position. 4 Conclusions Acetylation of starch with vinyl acetate using sodium carbonate as catalyst in an aqueous medium has been investigated. Degree of acylation was between 1.0 and 1.1, whereas a DS of only 0.02 was achieved for vinyl laurate (dodecanoate) under otherwise identical conditions. A reaction time of 5 h is sufficient for acetylation while 48 h were required to achieve DS 0.02 for the laurate. As vinyl esters are not miscible with water, the organic phase acts as a reservoir of the reagent. This prevents loss of vinyl esters by reaction with water so that equimolar amounts are consumed after recuperation of excess vinyl acetate. Acknowledgement We are indebted to the Bundesministerium fur Ver-braucherschutz, Ernahrung und Landwirtschaft (Fach-agentur Nachwachsende Rohstoffe, FKZ 99 NR 119) for financial support. References [1] A. M. Mark, C. L. Mehltretter: Facile Preparation of Starch Triacetates. Starke 1972, 24, 73-76. [2] I. A. Wolff, D. W. Olds, G. E. Hilbert: The acylation of corn starch, amylose and amylopectin. J. Am. Chem. Soc. 1951, 73, 346-349. [3] J. V. Tuschhoff, C. E. Smith: U.S. 3022289 (1962) (A. E. Staley Manufg. Co.). CA 56 (1962) 74378. [4] G. E. H. Joosten, E. J. Stamhuis, W. A. Roelfsema: Some Aspects of the Continuous Production of Low-Acylated Potato Starch. Starch/Starke 1982, 34, 402-405. [5] L. Mezynski, H. Strozycka, M. Buszka, G. Starogardzka: PL 159476 B1 (1992), Central Laboratory of Potato Industry, Poznan, PL; CA 122 (1995), 12428. [6] E. A. Klohr, W. Koch, D. Klemm, R. Dicke: EP 1035135 A1 (2000), Wolff Walsrode (Ger); CA 133 (2000) 224521. [7] R. Dicke, D. Klemm: Synthesis of Regioselectively Functionalized Polysaccharide Esters Avoiding Protecting Groups. Polymer Preprints 2000, 47,1550-1551. [8] J. Einfeldt, A. Kwasniewski, D. Klemm, R. Dicke, L. Einfeldt: Analysis of side group motion in O-acetyl starch using regioselective 2-O-acetyl starches by means of dielectric spectroscopy. Polymer 2000, 41, 9273-9281. [9] C. Deus, H. Friebolin, E. Seifert: Partiell acetylierte CelluloseSynthese und Bestimmung der Substituentenverteilung mit Hilfe der 1H-NMR-Spektroskopie. Makromol. Chem. 1991, 192, 75-83. (Received: July 26, 2003) (Revised: October 18, 2003) (Accepted: October 20, 2003)