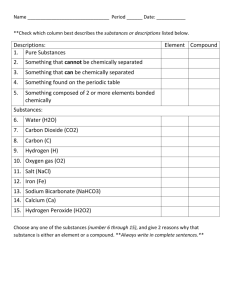
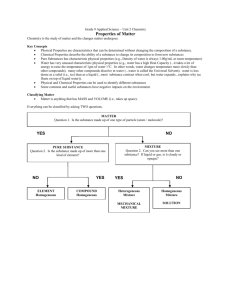
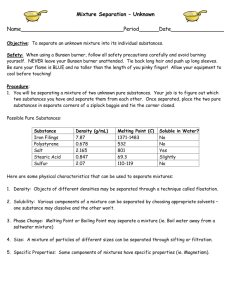
Substances/Mixtures
advertisement

Name: __________________________________ Date: _________________ Period: _____ Homework: Substances and Mixtures 1. A substance is made from a combination of simpler substances combined in a definite ratio. This substance is A an element B a compound C a mixture 2. A substance cannot be chemically separated into simpler substances. This substance is A an element B a compound C a mixture 3. A material is a combination of other substances that are not in a fixed ratio. The material can be physically separated into these simpler substances. This substance is A an element B a compound C a mixture Describe how you might separate the following mixtures based on their physical properties. 5. How would you separate a mixture of sulfur and iron filings? ___________________________________________________________ Sulfur and Iron Filings ___________________________________________________________ 6. How would you separate a mixture of salt and water? ___________________________________________________________ Salt and Water ___________________________________________________________ Identify each of the elements below based on the information given. 7. Which element has 8 protons, 8 neutrons and 10 electrons? ___________________________ 8. Which element has 15 protons and 16 neutrons? ____________________________________ 9. Which element has 3 protons, 4 neutrons and 3 electrons? ____________________________ 10. Which element has 12 neutrons and 11 protons? ___________________________________ The picture to the right shows a clear and blue liquid that have been placed in the same container and allowed to sit for 25 minutes. 11. Are these liquids miscible or immiscible? ___________________ 12. Why are these liquids not mixing? _______________________________________________ ______________________________________________________________________________ 13. Which liquid is most dense? ___________________________________________________ 14. What could you use to separate these liquids? _____________________________________ Answer the following questions about elements, compounds and mixtures. 1. A crystalline sample is brought into the lab. The laboratory technician notices that the sample can be chemically separated into simpler substances. The sample should be identified as – A an element B a compound C a mixture 2. A solid, silvery sample with a high melting point is brought into the lab for testing. The lab determines that the sample cannot be separated into substances. The sample is – A an element B a compound C a mixture 3. A metal sample with a broad melting point is brought to a lab. The lab determines that it can be separated into simpler substances by melting. The sample is – A an element B a compound C a mixture 4. A liquid sample is brought into the lab for testing. It is determined that the sample is a combination of simpler substances which have been joined together in a definite ratio, and that it can be chemically broken apart into these simpler substances. The sample is – A an element B a compound C a mixture 5. What type of material can be represented by dissolving large amounts of sodium chloride into a beaker of distilled water? A polymer B alloy C gaseous D aqueous solution 6. What type of material can be represented by melting samples of copper and zinc, mixing them together evenly and allowing them to cool and harden? A polymer B alloy C gaseous D aqueous solution 7. Air is a combination of different gases which do not exist in exact ratios and can be separated based on their physical properties. Based on this information, it can be inferred that air is a A an element B a compound C a mixture 8. Solutions are formed when substances are evenly mixed together. A solution would exist in which of the following phases? A solid B liquid C gas D solutions can exist in any phase Intensive vs. Extensive Properties Review 1. Are intensive or extensive properties based on the size of a sample? ____________________ 2. For each of the physical properties below, determine whether they are intensive (INT) or extensive (EXT). State of Matter _______ Number of Moles _______ Hardness _______ Density _______ Volume _______ Color _______ Odor _______ Temperature _______ Mass _______ Melting Point _______ Specific Heat _______ Energy _______ Boiling Point _______ Number of Atoms _______ Length _______