Minerals
Minerals!
Minerals are
naturally occurring, solid, crystalline substances with physical and chemical properties that vary within known limits. Although there are over 2000 minerals, we need to know only a few of them to identify most rocks.
Silicates
Nearly 75 percent by weight of the earth’s crust is oxygen and silicon . These two elements in combination with a few others ( aluminium, iron, calcium, sodium, potassium and magnesium) account for the chemical composition of minerals that make up about 95 percent of the earth’s crust. Minerals that include the elements silicon ( Si ) and oxygen ( O ) in their chemical composition are called silicates; these are the most abundant of the rock forming minerals . The three most important rock forming silicate minerals or mineral groups are quartz , feldspar , and ferromagnesian .
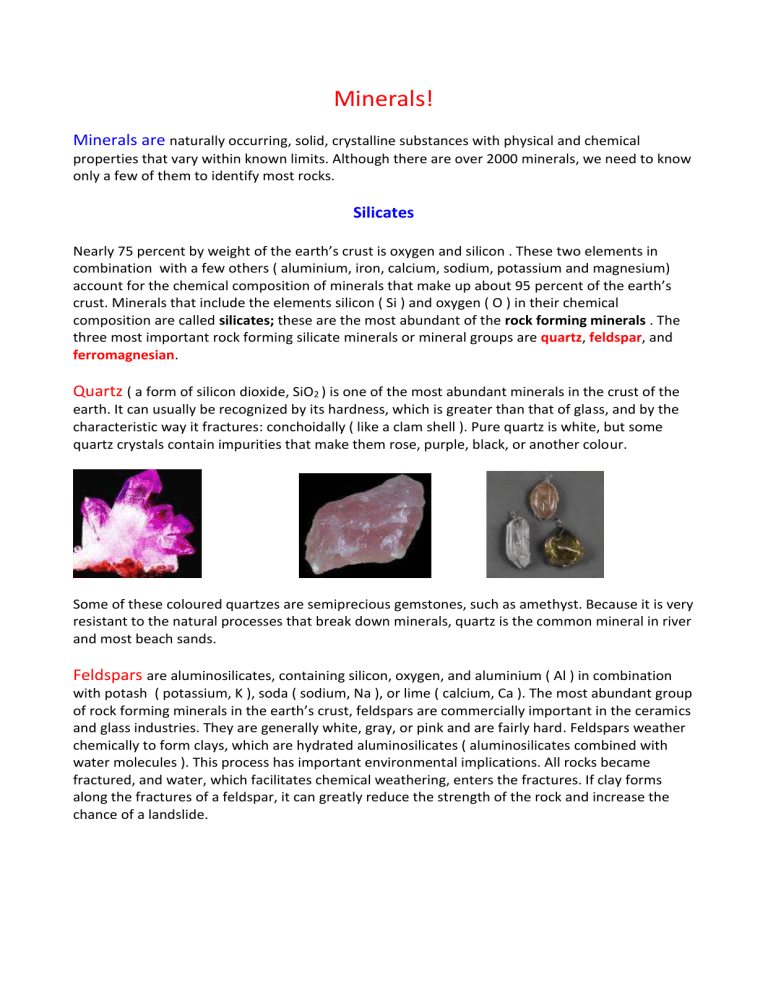
Quartz
( a form of silicon dioxide, SiO
2
) is one of the most abundant minerals in the crust of the earth. It can usually be recognized by its hardness, which is greater than that of glass, and by the characteristic way it fractures: conchoidally ( like a clam shell ). Pure quartz is white, but some quartz crystals contain impurities that make them rose, purple, black, or another colour.
Some of these coloured quartzes are semiprecious gemstones, such as amethyst. Because it is very resistant to the natural processes that break down minerals, quartz is the common mineral in river and most beach sands.
Feldspars
are aluminosilicates, containing silicon, oxygen, and aluminium ( Al ) in combination with potash ( potassium, K ), soda ( sodium, Na ), or lime ( calcium, Ca ). The most abundant group of rock forming minerals in the earth’s crust, feldspars are commercially important in the ceramics and glass industries. They are generally white, gray, or pink and are fairly hard. Feldspars weather chemically to form clays, which are hydrated aluminosilicates ( aluminosilicates combined with water molecules ). This process has important environmental implications. All rocks became fractured, and water, which facilitates chemical weathering, enters the fractures. If clay forms along the fractures of a feldspar, it can greatly reduce the strength of the rock and increase the chance of a landslide.
Ferromagnesian minerals
are a group of silicates in which the silicon and oxygen combine with iron ( Fe ) and magnesium ( Mg ). These are the dark minerals in most rocks ( for example the black mica biotite. Because they are not very resistant to weathering and erosional processes, ferromagnesian minerals tend to be altered or removed relatively quickly from their location. They also weather quickly, combining with oxygen to form oxides such as limonite ( rust ), and with other elements to form clays and soluble salts. These materials, when abundant, may produce weak rocks. Builders must be cautious when evaluating a construction site ( for example, for a highway, tunnel or reservoir ) that contains rocks high in ferromagnesians.
Other important rock forming minerals
In addition to silicates, rock forming minerals important in environmental studies include oxides , carbonates , sulphides , and native ( pure ) elements . Earth materials containing useful minerals ( especially metals ) that can be extracted are called ores . Iron and aluminium, probably the most important metals in our industrial society, are extracted from ores containing iron and aluminium
oxides. The most important iron ore is hematite ( Fe
2
O
3
) and the most important aluminium ore is bauxite ( Al
2
O
3
). Magnetite ( Fe
3
O
4
), also an iron oxide but economically less important than hematite, is common in many rocks. It is a natural magnet ( lodestone ) that attracts and holds iron particles. Where particles of magnetite are abundant, they may produce a black sand in streams or beach deposits.
Environmentally, the most important carbonate mineral is calcite ( Calcium carbonate, CaCO
3
).
This mineral is the major constituent of limestone and marble, two very important rock types.
Weathering of such rocks by water dissolves the calcite, often producing caverns, sinkholes ( surface pits ) and other unique features. In areas built on limestone, subsurface water in cavern systems may quickly become polluted by urban runoff. In addition, construction of highways, reservoirs, and other engineering structures is a problem where caverns or sinkholes are encountered.
The sulphide minerals, such as pyrite, or fool’s gold ( iron sulphide, FeS
2
), are sometimes associated with environmental degradation. This occurs most often when roads, tunnels, or mines cut through coal – bearing rocks that contain sulphide minerals. The exposed sulphides oxidize in the presence of water to form compounds such as sulphuric acid that may enter and pollute streams and other environments. This is a major problem in the coal regions of Appalachia and many other regions of the world where sulphuric coal and sulphide minerals are mined.
Native elements such as gold, silver, copper and diamonds have long been sought as valuable minerals. They usually occur in rather small accumulations but occasionally are found in sufficient quantities to justify mining. As we continue to mine these minerals in ever lower – grade deposits, the environmental impact will continue to increase. Large mines have a larger impact.