Limiting & Excess Reagents: Chemistry Notes
advertisement
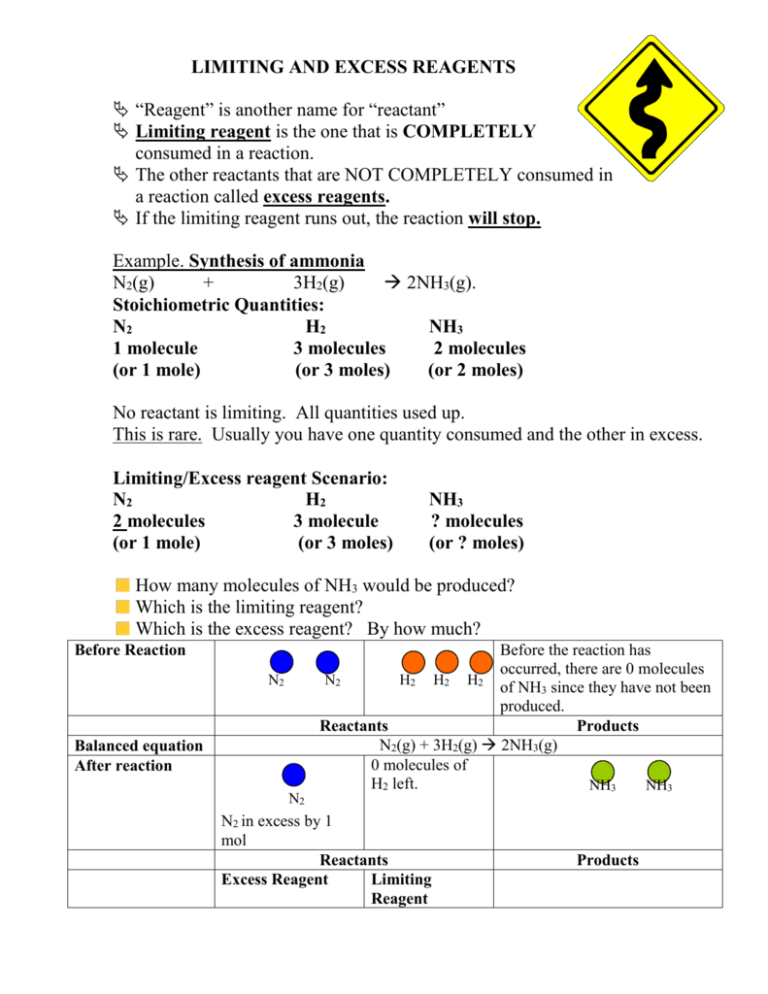
LIMITING AND EXCESS REAGENTS “Reagent” is another name for “reactant” Limiting reagent is the one that is COMPLETELY consumed in a reaction. The other reactants that are NOT COMPLETELY consumed in a reaction called excess reagents. If the limiting reagent runs out, the reaction will stop. Example. Synthesis of ammonia N2(g) + 3H2(g) 2NH3(g). Stoichiometric Quantities: N2 H2 NH3 1 molecule 3 molecules 2 molecules (or 1 mole) (or 3 moles) (or 2 moles) No reactant is limiting. All quantities used up. This is rare. Usually you have one quantity consumed and the other in excess. Limiting/Excess reagent Scenario: N2 H2 2 molecules 3 molecule (or 1 mole) (or 3 moles) NH3 ? molecules (or ? moles) How many molecules of NH3 would be produced? Which is the limiting reagent? Which is the excess reagent? By how much? Before Reaction N2 Balanced equation After reaction N2 Before the reaction has occurred, there are 0 molecules N2 H2 H2 H2 of NH3 since they have not been produced. Reactants Products N2(g) + 3H2(g) 2NH3(g) 0 molecules of H2 left. NH3 NH3 N2 in excess by 1 mol Reactants Excess Reagent Limiting Reagent Products What happened? 1 molecule of N2 reacted with 3 molecules of H2. There is still one more molecule of N2 left. However, the molecules of H2 have all been used up. The reaction can no longer continue since H2 is used up. H2 is the limiting reagent and N2 is the excess reagent. The quantity of the limiting reagent therefore determines ALL the quantities of the products formed in the reactions In limiting reagent questions we use the limiting reagent as the “given quantity” and ignore the reagent that is in excess … If we use the excess reagent, we will overestimate the amount of product that can be produced. Identifying the limiting reagent in a chemical reaction This is because the amount of limiting reactant that is available for a chemical reaction determines the amount of product that is formed and the amount of excess reactant that is left over. The limiting reagent yields the smaller amount of product in a chemical reaction. The limiting reactant forms less product - Limiting reactant is not necessarily the reactant this is present in the smaller amount (or smaller mass). It is the reactant that forms the smaller amount of product. e.g. 2H2(g) + O2(g)2H2O (g) If oxygen is present in excess and 2 mol of hydrogen is available, then 2 mol of water is produced (stoichiometric amounts) If hydrogen is in excess and 2 mol of oxygen is available then 4 mol of water is produced The moles of products are always determined by the starting moles of the LIMITING reagent Example 1: Sodium metal reacts with chlorine gas to form solid sodium chloride according to the following balanced equation: 2Na(s) + Cl2(g) 2NaCl If 4.80 mol Na reacts with 2.70 mol Cl2, determine the following: a) The limiting reagent b) The mass of excess reagent that remains unreacted. c) The amount of NaCl that is produced Step 1: write a balanced equation: Already done for you: 2Na(s) + Cl2(g) 2NaCl Step 2: Analyze the mole ratios/proportions. The equation tells us that 2 moles of Na will react with 1 mole of Cl2 to produce 2 moles of NaCl. Step 3: Check the reagent ratios to see if they are correct according to the balanced equation ratio. 4.80 mol Na will react with how many mole Cl2? 4.80 mol Na x 1 mole Cl2 = 2.40 mole of Cl2 2 mole of Na The question tells us that there are 2.70 mole of Cl2. Which means that Cl2 is in excess since 2.70 > 2.40 (the actual amount that 4.80 mole of Na would react with). Therefore, Na(s) is the limiting reagent. Excess mol Cl2= 2.70 -2.40 mol = 0.30 mol Excess mass of Cl2 that remains unreacted: mcl2= 0.30 mol x 71 g/mol=21.3 g Rule: 1. If Mole stated in question > calculated mole, then that is your excess reagent. 2. If Mole stated in question < calculated mole, then that is your limiting reagent. To calculate the amount of NaCl produced: Use the mole amount of the limiting reagent in order to calculate amounts of any other substance in the equation. Calculate the amount of ammonia that is produced using the moles of the limiting reagent. 4.80 mole Na x 2 mole NaCl = 4.80 mole of NaCl produced. 2 mole Na If you used the other value? 2.70 mole Cl2 x 2 mole NaCl = 5.40 mole of NaCl produced. 1 mole Cl2 If you calculated using excess reagent, the amount of product would mistakenly be too high! Method 2: a second method to determine limiting reagents Example 2: The chemical compound ammonia is prepared from its elements according to the following chemical equation:N2(g) + 3H2(g) 2NH3(g) If 4.20 g of nitrogen gas reacts with 0.750 g of hydrogen gas, which is the limiting reactant? Plan your strategy Calculate the molar masses, M, of nitrogen and hydrogen Convert the masses of nitrogen and hydrogen into amounts (in moles) using n = m/M Calculate the amount of ammonia that is produced by the given amount of nitrogen and the given amount of hydrogen (using mole ratios). Compare the amounts of ammonia that are produced by nitrogen and hydrogen to determine the limiting reactant. Act on your strategy MN2 = 2 x MN = 2 (14.01 g/mol) = 28.02 g/mol MH2 = 2 x MH = 2(1.01 g/mol) = 2.02 g/mol nN2 = mN2/MN2 = 4.20 g/28.02 g/mol = 0.14989 mol of N2 nH2 = mH2/MN2 = 0.750g/2.02 g/mol = 0.37129 mol of H2 nNH3 = 0.14989 mol N2 X 2 Mol NH3 = 0.300 mol NH3 1 mol N2 nNH3 = 0.37129 mol H2 X 2 mol NH3 = 0.248 mol NH3 3 mol H2 The calculation with H2 gas produces less NH3 than N2. Therefore Hydrogen gas is the limiting reagent despite there being more moles of Hydrogen gas present! DO NOT ASSUME THAT THE LIMITING REAGENT IS THE REAGENT PRESENT IN THE SMALLEST QUANTITY! IT IS ALL ABOUT THE MOLAR RATIOS! Adjusted Approach for Limiting Reagent Problems KEY: If 2 masses are given (ie. the mass of both reactants), then the question is a limiting reagent problem. 1) Balance the given equation 2) a) Convert the quantity of the given substance to moles of the given substance b) Determine limiting reagent 3) Using the moles of the limiting reagent to determine the moles of the unknown substance (use mole ratio derived from balanced equation) 4) Convert the moles of the unknown to the desired quantity of the unknown. **In any of the above cases, the mass calculated will be a theoretical mass. This mass may then be used to determine the percentage yield.








