Experiment M02
advertisement

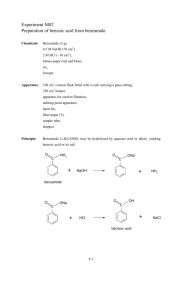
Experiment M02 Preparation of benzoic acid from benzamide Name: Seat No.: Date: Grade: Results Colour of crystals obtained: White Shape of crystals: Needle-like Melting point of the crystals: 122 oC Mass of benzamide used: 3.03 g Mass of crystal product obtained: 1.72g Molecular mass of benzamide (C6H5CONH2) = 121 Molecular mass of benzoic acid (C6H5COOH) = 122 1.72 Percentage yield = 122 × 100% = 56.30% 3.03 121 P.3 Experiment M02 Preparation of benzoic acid from benzamide Interpretations 1. Is benzamide soluble in (i) water, (i) 2. (ii) aqueous NaOH? Slightly soluble (ii) Yes Write equations for the reactions involved in procedure b. O C NH2 O ONa C + NaOH + NH3 benzamide 3. After boiling for 20 minutes, what is the appearance of the reaction mixture? Pale yellow ppt is observed. 4. In step c, how can you tell when the acid has been added in excess? The mixture turns blue litmus paper red. 5. What is the purpose of washing the crystals in step d? Why is the amount of water used kept at a minimum and kept cold? To wash away the impurity adheres on the crystals. Prevent the lost of the product. (Solubility decrease as the temperature of water decrease) 6. The melting point obtained by students is usually lower than the true value. What may be the reason(s) for this? What improvements could be made? As the crystals are not pure and contain some impurities. This may be improved by recrystalize the crystals more than 1 time and wash the crystal several times. 7. What criteria are usually used to judge whether a preparation method is good. 1. The yield is high. 2. High purity. 3. Economical. 4. Rate of reactions is fast. 5. Involve a few steps. P.4










![N, N'-1, 2-Phenylenebis [4-(chloromethyl) benzamide]](http://s3.studylib.net/store/data/008118148_1-ef645986f4e09c3026821856b13578f0-300x300.png)