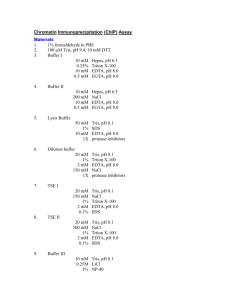
RRECIPES for buffers

RECIPES for buffers
Common Saline solutions:
Ca-free Tyrode’s solution:
140mM NaCl
5mM KCl
10mM HEPES
2mM BAPTA
10mM glucose
10mM Na pyruvate adjusted to pH 7.4 with NaOH
Phosphate-buffered saline (PBS)
Typical working concentration for perfusion: 0.01M phosphate-buffered saline (pH 7.4, room temperature)
10X Stock Solution, 1 Liter
80 g NaCl
2 g KCl
11.5 g Na
2
HPO
4
-7H
2
O
2 g KH
2
PO
4
Working stock, pH ~7.3
137mM NaCl
2.7mM KCl
4.3mM Na
2
HPO
4
-7H
2
O
1.4mM KH
2
PO
4
1X TTBS
Add 6.05 g Tris base (50 mM), 8.76 g sodium chloride (150 mM) to 800 mL of distilled water.
Adjust pH to 7.5 with HCl. Adjust to 1 liter with distilled water. Add Tween-20 to 0.1% (v/v). Maybe stored for up to 3 months at room temperature.
To make 1.0 Liter of TTBS:
800 mL of deionized water
Add 12.1 g of Tris base
Add 8.8 g of NaCl
Dissolve and adjust pH to 7.5 with HCl
Add 1.0 mL of Tween-20 (provided)
Bring volume up to 1.0 Liter with deionized water
Filter with 3 mM Whatmann paper or equivalent
Store at room temperature for no more than one month
To make 100 mL of TTBS with 1.0% (w/v) BSA:
100 mL TTBS
Add 1.0 g of BSA (provided)
Dissolve and use immediately
RECIPES
Lysate Buffer (Plasmid Prep Solution #1: 0.1 M NaCl, 50 mM Tris-HCl pH 7.8-8.5,
10 mM EDTA)
For 100 ml, add 2 ml of 5 M NaCl, 5 ml of 1 M Tris-HCl stock at the appropriate pH,
2 ml 0.5 M EDTA and Q.S. with distilled water. Autoclave.
5% Nonfat Dry Milk
Prepare using Carnation Nonfat Dry Milk in TBST.
10% Ammonium Persulfate (APS) Solution
0.12 g APS
1.2 ml H
2
O)
Keep for no more than week in the refrigerator.
TBST (10 mM Tris-HCl, 0.15 M NaCl, 8 mM sodium azide, 0.05% tween-20, pH
8.0)
Chemical For 4 liters
Tris 4.84 g
For 500 ml
0.61 g Tris
NaCl
NaN
3
Tween-20
35.06 g
2.0 g
2.0 ml
4.38 g NaCl
500 µl 10% NaN
3
250 µl Tween-20
Adjust pH to 8.0 with HCl. QS with water and store at 4°C.
Tank Buffer (25 mM Tris-HCl, 0.2 M glycine, 0.35% SDS)
Chemical For 4 liters
Tris 12.11g
Glycine 57.6 g
For 500 ml
1.51 g Tris
7.2 g
SDS 4.0 g 17.5 ml 10% SDS
QS with H
2
O. pH should be 8.3 but adjusting is not needed. Store at 4°C
Transfer Buffers:
Wet blot transfer buffer (3L) (25 mM Tris-HCl, 0.2 M glycine, 20% methanol).
9.08 g Tris
43.24 g glycine
600 ml methanol
QS to 3 L with water.
Dry blot transfer buffers
Stock Solution A1 (3 M Tris)
36.3 g Tris, dissolved in double distilled water and QS to 100 ml
Store at room temperature.
Stock Solution A2 (0.3 M Tris)
3.63 g Tris, dissolved in double distilled water and QS to 100 ml
Store at 4°C.
Stock Solution C (0.3 M Tris, 0.4 M aminohexane)
3.63 g Tris, 5.2 g aminohexane, dissolved in double distilled water and qs to to
100 ml.
Store at 4°C.
Dry Blot Working Solutions (made from fry blot transfer buffers)
7 ml H
2
0
2 ml Methanol
1 ml stock solution of A1, or A2 or C
Store at 4°C.
Lower Gel Buffer (1.5 M Tris-HCl, 0.4% SDS, pH 8.8)
For 100 ml:
18.17 g Tris
4 ml 10% SDS
Adjust pH to 8.8 with HCl. QS to 100 ml with water. Store at room temperature.
Upper Gel Buffer 0.5 M Tris-HCl, 0.4% SDS, pH 6.8)
For 100 ml:
6.06 g Tris
4 ml 10% SDS
Adjust pH to 6.8 with HCl. QS to 100 ml with water. Store at room temperature.
4x Sample Buffer (made from stock sample buffer) (0.24 M Tris-HCl, 0.24 M SDS,
40% glycerol, 20% 2-mercaptoethanol, pH 6.8)
8 ml of stock sample buffer
2 ml of concentrated 2-mercaptoethanol
Transfer to 1 ml aliquots and store at -20°C.
2x Sample Buffer (Hoeffer Scientific) (125 mM Tris-HCl, 4% SDS, 20% glycerol,
10% 2-mercaptoethanol, 0.004% bromophenol blue).
Prepare in fume hood!
2.5 ml 0.5 M Tris-HCl, pH6.8 and 4.0 ml of 10% SDS
(--or-- 2.5 ml of upper gel buffer and 3.6 ml of 10% SDS)
2.0 ml glycerol
1.0 ml concentrated 2-mercaptoethanol
0.4 mg bromophenol blue (you may use less but don't add more than 0.4 mg)
QS to 10 ml with water. Transfer to 1 ml aliquots and store at -20°C.
Stock Sample Buffer (from Craig Wasson) (0.312 M Tris-HCl, 0.346 M SDS, 50% glycerol, pH 6.8)
3.03 g Tris
8.0 g SDS
40 ml glycerol
Adjust pH to 6.8 with HCl. QS to 80 ml with water. Store at room temperature.
0.1% Fast Green (in 10% acetic acid and 20% methanol)
0.2 g Fast Green
20 ml acetic acid
40 ml methanol
140 ml double distilled water.
0.1% Amido Black (in 10% acetic acid and 45% methanol)
0.2 g Buffalo Black
90 ml methanol
20 ml acetic acid
90 ml double distilled water.
Amido Black Destain (2% acetic acid, 45% methanol)
225 ml methanol
10 ml acetic acid
QS to 500 ml with double distilled water.
Useful formulations for Western blotting and
Immunoprecipitation
Cell Lysis Buffer
50 mM Tris-HCl, pH8.0
150 mM NaCl
1% NP-40 or Triton® X-100
2x Immunoprecipitation Buffer
2% Triton® X-100
300 mM NaCl
20 mM Tris, pH 7.4
1.0% NP-40
2 mM EDTA
2 mM EGTA
0.4 mM sodium vanadate (phosphatase inhibitor)
0.4 mM PMSF
2x SDS-PAGE Sample Buffer
100 mM Tris-HCl, pH 6.8
2% Sodium Dodecyl Sulfate (SDS)
20% Glycerol
0.2% Bromophenol Blue
2–10%
-mercaptoethanol (or DTT)
100x Protease Inhibitor Cocktail
PMSF 5 mg (50 μg/mL)
Aprotinin 100 μg (1.0 μg/mL)
Leupeptin 100 μg (1.0 μg/mL)
Pepstatin 100 μg (1.0 μg/mL)
Add 100% Ethanol to 1 mL. Aliquot and store at -20°C.
Recommended Acrylamide Gel Percent
Recommended % Acrylamide
8%
10%
12%
Protein Size Range
40–200 kDa
21–100 kDa
10–40 kDa
Coomassie Staining
0.2% Coomassie Gel Stain (in 7.5% acetic acid and 40% methanol):
0.2 g Coomassie
7.5 ml acetic acid
40 ml methanol
Q.S. with H2O to 100 ml. Nalgene or Whatman filter before use.
Coomassie Rapid Destain (7.5% acetic acid and 5% methanol):
75 ml acetic Acid
50 ml methanol
Q.S. with H20 to 1 L.
Silver Staining (Charles O'Brien)
Fixative 1
40% methanol
10% acetic acid
Prepare 500 ml
Fixative 2
10% ethanol
5% acetic acid
Prepare 1L
10x Oxidant
2.5 grams potassium dichromate (0.034M)
500
l nitric acid (0.032M)
QS to 250 ml with double distilled water. Store at 4
C for up to one year.
Make sure the working dilution is at room temperature before use.
10x Silver Nitrate
5.10 grams silver nitrate (0.12M)
QS to 250 ml with double distilled water. Store at 4ºC for up to one year.
Make sure the working dilution is at room temperature before use.
Developer (make fresh each time)
29.68 grams sodium carbonate (0.28M)
0.5 ml formalin (formaldehyde)
QS to one liter with double distilled water. Make sure the solution is at room temperature before use.
Stop solution
5% acetic acid