A complete beginner`s guide to attacking bacteria
advertisement

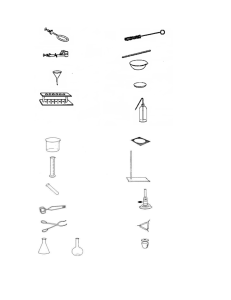
Core Practicals for Year 11 Science Teachers' and Technicians' notes by Anne Wignall and Terry Wales 1 Precipitation reactions A simple precipitation experiment to try to find out which compounds are soluble and which insoluble. Equipment spotting tiles (or test tubes in racks) droppers Chemicals Approximately 0.1 mol L–1 solutions (concentration is not critical) of NaCl, Na2SO4, NaOH, Na2CO3, KNO3, Cu(NO3)2, Mg(NO3)2, Ca(NO3)2, Pb(NO3)2, Fe(NO3)2 (or FeSO4 labelled Fe2+, if iron(II) nitrate is not available). 2 Neutralisation reactions Many students believe that acids react with everything. This practical is intended to help students understand that only some substances will react with and neutralise acids. Students may already know the gas given off when acids react with carbonates, or you may choose to have them test for hydrogen and oxygen as well. Equipment spotting tiles (or test tubes in racks) droppers delivery tubes and matching test tubes large beaker or ice-cream container burner (if testing for oxygen and hydrogen) wooden splints (if testing for oxygen) Chemicals bench solutions of HCl and H2SO4 (1 or 2 mol L–1) two solid chlorides eg NaCl, KCl, MgCl2, ZnCl2 two solid carbonates eg Na2CO3, K2CO3, CuCO3, ZnCO3, MgCO3 two solid hydroxides eg Ca(OH)2, Mg(OH)2, Zn(OH)2 two solid nitrates eg NaNO3, KNO3, Mg(NO3)2, Zn(NO3)2 two solid oxides eg MgO, ZnO, CuO two solid sulfates eg Na2SO4, K2SO4, MgSO4, ZnSO4, CuSO4 universal indicator solution limewater 3 The reactivity of metals The relative reactivity of common metals with oxygen, water and acid is compared. Students should not work with the reactive metals (sodium and lithium)—and teachers should only work with half-rice-grain sized samples of these metals. Students need to take care when working with calcium, which should not be reacted with acid. SAFETY GLASSES MUST BE USED FOR THIS EXPERIMENT. Note: if you put a small piece of aluminium foil in a beaker containing 10 mL of dilute HCl with a teaspoon of NaCl dissolved in it, and leave it for 10 minutes, the metal will begin to react with the acid. The reaction is relatively vigorous, but noticeably less vigorous than with magnesium. If you leave out the salt the aluminium reacts with HCl after about 20 minutes. Equipment watch glasses (or ceramic tiles or other alternative) sandpaper Bunsen burners tongs Chemicals powdered metals eg Mg, Al, Zn, Fe, Cu metal foil or strips eg Mg, Al, Zn, Cu, steel wool teacher demonstration only: Na, Li universal indicator solution bench (1–2 mol L–1) HCl or H2SO4 solid NaCl (optional) 4 Magnesium and steam This is a teacher demonstration. The reaction will only proceed if the magnesium gets hot enough. If you have a hot enough flame you can use a Pyrex test tube, but with most gas supplies a thin-walled (disposable) tube will be needed. The test tube usually softens and may even bend during the reaction (which adds to the entertainment value). Once the magnesium ignites the heat is sufficient to melt the glass anyway. WEAR SAFETY GLASSES. Equipment disposable test tube rubber bung with short glass tube (to fit test tube) clamp and stand cotton wool newspaper sandpaper red litmus paper Bunsen burner hammer Chemicals 5 cm strip of magnesium ribbon 5 Metal and non-metal oxides Magnesium oxide and sulfur dioxide are used to compare the properties of metal and non-metal oxides. If possible a fume cupboard should be used to burn sulfur. The reaction is more spectacular done in a gas jar of oxygen. Equipment tongs Bunsen burner red litmus paper watch glass, evaporating dish or small beaker gas jar deflagrating spoon chemicals magnesium ribbon sulfur powder oxygen (optional) universal indicator solution 6 Copper compounds The decomposition of copper carbonate is a common examination topic. Students are expected to remember the colour changes that take place. The ‘copper carbonate’ powder we use is actually a mixture of copper carbonate and copper hydroxide, so when it is heated significant amounts of water vapour are formed. That is why it is important that students tilt the test tube so it is horizontal or sloping slightly downwards. (Also remember to get them to cool the test tube before adding cold acid!) Equipment test tube holders Pyrex test tubes Bunsen burner Chemicals display samples of these solids: copper metal, Cu2O, CuO, CuCl2, CuCO3, CuSO4 (hydrated) copper carbonate powder for heating spatula bench H2SO4 (1–2 mol L–1) 7 Particle size and reaction rate Use this practical to discuss fair testing. What factors are being controlled? Why? What is the factor that is being changed? A more quantitative measure of reaction rate could be made by adding a few drops of detergent and measuring the height of bubbles formed after 30 seconds. Equipment test tubes spatulas scrap paper beaker (for water bath) a balance, if you wish to weigh out the calcium carbonate Chemicals marble chips CaCO3 powder bench HCl (1–2 mol L–1) 8 Concentration and reaction rate Another opportunity to discuss fair testing while investigating the way concentration influences the rate of reaction. Teachers could get students to plan this experiment before following these instructions. Note: you may prefer to start with more concentrated HCl so the time taken for each reaction is faster. Do a test run with your acid and magnesium ribbon first. Equipment test tubes and rack sandpaper (optional) scissors (optional) 10 mL measuring cylinders or 2 mL droppers beaker for water bath clock with a second hand or stopwatch (if students don't have watches) Chemicals magnesium ribbon bench HCl (1–2 mol L–1) 9 Testing catalysts This practical gives students a chance to compare the rate of a reaction without, and with, a catalyst. The reaction mixture should be discarded quickly after it turns colourless, because another reaction occurs which produces small amounts of SO2 gas. Cu2+ is the catalyst for this reaction. If the other ions are not available you could substitute other transition metal salts such as Ag+ or Zn2+. Fe3+ + 2S2O32– [Fe(S2O3)2]– (violet complex) rapid reaction [Fe(S2O3)2]– + Fe3+ 2Fe2+ + S4O62– slow reaction, catalysed by Cu2+, tungsten and selenium. (See Vogel's Textbook of Macro and Semi-micro Qualitative Inorganic Analysis.) Note: it is important to acidify the Fe3+ solution. Lemon yellow Fe3+ will turn colourless, but orange Fe3+ returns to orange after the dark complex has reacted, making an exact end-point almost impossible to determine. Equipment 100 mL beakers 10 mL measuring cylinders (or 25 mL) droppers Chemicals Main reagents (50 mL of each needed for each group): dilute (approx 0.1 mol L–1) sodium thiosulfate (Na2S2O3) dilute (approx 0.1 mol L–1) Fe(NO3)3 or FeCl3. Acidify this with a few drops of dilute HCl so that the liquid is lemon yellow rather than orange. Catalyst reagents (one drop needed per group): solutions (approx. 0.1 mol L–1, concentration is not critical) of Fe2+, Ni2+, Cu2+, and Co2+ (sulfates, chlorides or nitrates are all suitable). 10 Experimental design: temperature and reaction rate Having investigated fair tests involving particle size and concentration, students are now invited to plan their own fair test to investigate the affect of temperature on the rate of reaction. This investigation could be used as the basis for NCEA task Science 1.1. Note: if students are doing this as an individual, assessed practical they will need a stopwatch. Equipment beakers thermometers test tubes hot water (Bunsen or kettle) sandpaper (optional) clock with a second hand or stopwatch (if students don't have watches) Chemicals magnesium ribbon bench HCl (1–2 mol L–1) 11 Model manipulation Building models of organic compounds helps students understand how it is possible to have many different compounds with the same formula, and how carbon can form single, double or triple bonds (but not quadruple bonds). It also helps them to get a feel for the three-dimensional shape of the molecules. Equipment Organic model kits. Each group needs a minimum of 4 carbon (black) and 10 hydrogen (white) atoms. 12 Properties of alkanes This experiment is most conveniently done as a class demonstration. Equipment test tubes and racks electrical conductivity testing kit (the physics or chemistry staff may have a purpose-built kit, or you can make your own using a power supply, bulb and some leads) evaporating dish or crucible (for igniting alkanes) string (for wick for higher-mass alkanes) matches gas jar or large glass beaker cobalt chloride paper white tile or spotting tray Chemicals selection of alkanes such as pentane, hexane, cyclohexane, octane, petrol, kerosene, diesel, paraffin wax, paraffin oil candle limewater 13 Experimental design: combustion Many students will have done an investigation like this in Year 9, but it is worth repeating to remind them of the characteristics of a Fair Test. They need to practise writing down the obvious—such as use the same burner for each trial. It is also worth discussing the chemical differences between complete and incomplete combustion. Equipment Bunsen burner tripod and gauze mat beakers thermometers clock 14 Combustion of alcohol Students have difficulty drawing diagrams involving delivery tubes correctly. This experiment is more about the correct arrangement and purpose of the various tubes than it is about the combustion products of alcohol. Equipment meths burner delivery tubes, stoppers and test tubes as in diagram filter pump (for vacuum) beaker Chemicals meths limewater cobalt chloride paper ice 15 Fractional distillation Many students will have seen a distillation set-up in Year 9, but it is worth repeating this year both as a reminder and for those students who did not understand what they saw back then. The thermometer can be used to show that the ethanol boils off at 78 °C. Equipment distillation glassware as shown. cobalt chloride paper teaspoon or small crucible Chemicals methylated spirits (or ethanol) and water mix (1:2). 16 Revolutionary revision Students should know all of these—but some will need reminding. If possible, do this experiment in a darkened room. (You may find it darker in the corridor, or even on the stage with the curtains drawn.) Equipment small light source such as a 12 V bulb or candle (it helps to elevate the light on a tripod) tripod or box to elevate light large ball (such as a tennis ball, orange, baseball, polystyrene ball etc) small ball, such as a ping pong ball, plum, golf ball, marble, etc (if all else fails, screw sheets of paper into balls!) 17 Moon-watching Year 11 students are not known for their willingness to carry out observations over a period of two weeks, but it is a worthwhile exercise even if only a few of your class complete it. Not only do they get a better understanding of the phases of the moon, but they will be making other astronomical observations, such as noticing the changing pattern of the stars over that time period. If you start making observations around the time of new moon then the moon will be visible in the early evening. The Carter Observatory site: www.carterobs.ac.nz gives the phases of the moon for each month, plus planets that will be visible. Equipment A few cloudless nights! 18 The expanding universe In theory you can do this experiment using a balloon, but it needs to be a good quality balloon that inflates evenly in all directions. Equipment a ruler that measures in mm a calculator 19 Seasons in the sun The difference made by the altitude of the sun in summer and winter to the amount of light energy received by a square metre of land is clearly seen in these calculations. Equipment ruler scientific calculator (one that has trig functions) 20 Mineral identification Kits of common minerals are available from a variety of sources, including the University of Otago Geology Department. With less able classes you will probably need to do this practical as a class demonstration, but it is worth doing to give the students an understanding of the processes used in mineral identification. If students are doing this experiment themselves, you will find it easiest to set up four stations around the room. (Note: two samples of galena will be required.) Station one: streak a white unglazed tile (either an official 'streak' tile, or the back of a spotting tray) samples of haematite, galena, chalcopyrite and rhodonite Station two: hardness a piece of glass, eg a disposable test tube, or jam jar a steel knife or sharp nail samples of quartz, gypsum, sulfur and fluorite Station three: form good crystals of muscovite, pyrite, galena and sphalerite Station four: chemical tests small beaker or watchglass distilled water in a wash bottle (or in a beaker with a dropper) dilute silver nitrate solution with dropper (caution: stains clothes—wash off immediately if spilt) dilute (bench) hydrochloric acid solution with dropper tissue paper to dry crystals samples of calcite, halite, barite and halloysite 21 Rock identification Most schools have collections of rocks, but if yours doesn't, you can buy kits with samples of common rocks from rock shops or from Otago University. Labelling rocks can be a challenge as sticky labels often fall off. A blob of white correcting fluid, followed by a Vivid marker, is usually successful. (Numbers are easier to distinguish than letters.) Alternatively, consider using different colours of paint or nail varnish to label the samples. Equipment A variety of rocks from the following choice: chalk, limestone, conglomerate, breccia, sandstone, shale, mudstone, clay, greywacke, rhyolite, basalt, granite, gabbro, gneiss, schist, pumice, scoria, coal, obsidian, marble, quartzite and slate. (The underlined rocks are particularly important; students should be given a mix of sedimentary, igneous and metamorphic rocks.) dilute HCl plus a dropper (students should be told to rinse off acid and dry rocks after testing) tissue paper to dry rocks hand lens (optional) 22 Clean water This experiment may be done while discussing the environmental impact of mining, or as part of an investigation into metal compounds. It is best done as a demonstration. Equipment beaker dropper test tube Chemicals FeSO4 solution (concentration unimportant) CuSO4 solution (concentration unimportant) limewater concentrated ammonia solution plus dropper 23 Growing microbes It's easy for teachers to forget the wonder students feel when they see microbes they sampled themselves growing on an agar plate. It's also easy for teachers to be somewhat blasé about the risks involved in culturing microbes at blood heat. Samples should not be taken from locations such as the gym floor or handbasins where human pathogens are likely to be found, and plates should be incubated at 25 °C, rather than 37 °C. Equipment sterile (ie unused) cotton buds agar plates nutrient agar: To 1 L of water add 15 g of agar, 5 g of peptone, 5 g of beef extract (oxo cubes) and boil for 15 minutes. Pour into sterile Petri dishes while still liquid and place lids on dishes immediately. Sellotape to seal plates (or cling-film from a roll that has been cut into 2 cm wide rolls) incubator or warm room 24 Attacking bacteria Traditionally this experiment is done using a culture from the previous experiment. There is an element of risk involved, since you do not know whether the culture selected is a human pathogen or not. A much safer alternative is to contact your local polytech or hospital. They may be able to give you a sample of a known bacterium to work with. (Remember to ask them for the correct nutrient recipe for the bacterium supplied, and the incubation temperature.) Glasses, face masks and gloves should be used, and the inoculation of the plates is best done as a demonstration. (See A complete beginner's guide to attacking bacteria at the end of this document.) Photographs of our plates are included in the Results file. As an alternative to testing different brands of disinfectant, you may choose to test different dilutions of the same brand. Equipment bacterial culture agar plates suitable for the culture used nutrient broth (ie nutrient agar minus the agar itself) suitable for the culture used wire loop (if not available use a sterile toothpick) sterile cotton swabs (these can be made by wrapping a small amount of cotton wool on the end of a kebab stick, or you could use sterile cotton buds instead) filter paper pencil (2B or darker) or OHP pens to label Petri dishes directly scissors disinfectant/antiseptic solutions sterile water small beakers measuring cylinders or graduated pipettes 2 or 3 metal tweezers alcohol solution and flame source (Bunsen or meths burner) tissue paper (optional) antibiotic discs or polydisc (if available) incubator (we had good results by using a chilly bin warmed with a 2 L milk bottle filled with hot tap water) 25 Fermentation trials This experiment uses a control to establish the conditions needed for fermentation. Equipment boiling water (kettle or Bunsen) thermometer 3 flasks and delivery tubes as shown in diagram Distillation glassware as shown in diagram: Chemicals sugar or glucose granulated yeast limewater 26 Continuous variation This practical rapidly creates a bar chart of the heights of students in the class. Note that in the first (2001) printing of the student practical book the values for 165– were inadvertently omitted. Students should renumber as appropriate for the data collected. Equipment large sheet of paper (minimum A3 size) metre rule or tape-measure marker pen or crayon pins or tape to stick sheet to wall 27 Human genetics It's fun to look at the variety of characteristics within a class and to guess which ones are dominant and which recessive based on your class's results, but of course it isn't really valid. Some genes (such as that for Huntington's disease) are very rare, but dominant when they are present. People living in certain towns sometimes have particular genes in common which are rarely found elsewhere. Once the class has investigated these characteristics, compare their frequency to this table and discuss the validity of the exercise. Chin dimple Earlobes Eyebrow texture Eyebrow length Freckles on face Lip thickness Cheek dimples Hair on middle segment of fingers Hairline Little finger Tongue rolling Thumb Dimple dominant Free earlobes dominant, attached recessive Bushy dominant, fine recessive Connected recessive Facial freckles dominant Thick dominant, thin recessive Dimples dominant Hair present dominant, none recessive V-shaped (widow's peak) dominant Bent dominant, straight recessive Rolling dominant Straight dominant, curved recessive Equipment None required 28 The principles of genetics This exercise is intended to help students apply the various words and concepts used in this topic. It is useful early on in the topic, but after they have considered the idea of dominant and recessive characteristics. Through it they begin to understand that genes are carried on chromosomes, that chromosomes come in pairs, that gametes carry only one chromosome from each pair, that two gametes are joined to make a new individual and how sex-linked characteristics can be carried by females but appear in males. They also get lots of practice in predicting what phenotype will be seen with a particular genotype. If you prefer, you can create your own chromosomes. A pack of cards is useful for generating a random mix of dominant and recessive alleles—red cards for dominant (capital letter) and black for recessive (lower case letter). Note: a wererabbit is an individual which turns into a rabbit when the Moon is full. Equipment None required 29 Growth trial Although this practical looks time-consuming, it is worth doing because it gives students practice in planning and carrying out a Fair Test and also allows them to consider aspects of Plant and Animal Management. If all the plants are kept together they can be quickly watered by one student (or teacher). Do take the time to weight the plants at the end of the experiment: it gives students practice in weighing, and it emphasises the need for quantitative results. Many variations of seeds, growing medium and conditions are possible. Here is one that has worked for us. Half fill 2-litre icecream containers with river sand. Mix 0, 0.5 g, 1 g, 1.5 g, 2 g or 2.5 g of granular lawn fertiliser into each one. Plant 5 barley seeds into each container (no more). Keep the sand moist throughout the growing period. Make sure all the plants receive the same amount of light—you many need to rotate the containers each day. The hardest part about this practical is making sure other students do not interfere with the plants. It takes almost 2 weeks for the seeds to germinate, and fertiliser concentration has no effect on germination rates, so you may find it easier to germinate the seeds in advance, and have the students plant out 5 seedlings per container. Equipment river sand or powdered pumice barley seeds all purpose lawn fertiliser balance icecream containers 30 Switching on to circuits Students often have difficulty converting to and from real circuits and circuit diagrams. Equipment power source (cells or power supply) bulbs suitable for power source—a minimum of three bulbs per group wires switches if available 31 Current in series and parallel circuits Students should be shown the correct way to connect an ammeter in a circuit, and also taught to 'touch test' before they complete the circuit. This practical works best with the higher currents drawn from a power supply, rather than cells. If you are using dual scale meters, you will also need to explain how to read the meter. Equipment power source bulbs suitable for power source—a minimum of two bulbs per group wires ammeter (one per group) 32 Voltage in series and parallel circuits Students will need to be shown the correct way to connect a voltmeter in a circuit and taught to 'touch test' before completing the circuit. Equipment power source bulbs suitable for the power source—a minimum of two bulbs per group wires voltmeter (one per group) 33 Ohm's law This experiment turns up in almost every examination paper, so it is worth students carrying this out themselves. Equipment power supply ammeter voltmeter rheostat (variable resistor) coil of resistance wire (7–15 W) wires alligator clips if necessary beaker ice 34 Diodes Diodes have not appeared in the most recent School Certificate examinations, but are mentioned in the explanatory notes for Science 1.6, as well as in the Curriculum document. It is probably a good idea to give your students a chance to work with diodes if you have them. A common misconception of students is that a bulb will glow if it appears before a diode facing the wrong way, but not if the bulb comes after the diode. Equipment cells or power supply bulb suitable for power source wires switch (optional) diodes or LEDs 35 Graphing a journey It is worth taking the 10 minutes or so of class time needed to generate results for this experiment because it gives students a feel for the concepts involved. Equipment tape-measure or string marked in 10 m intervals stopwatches (10) 36 Testing ticker-timers Ticker-timers may be 'old hat' to teachers, but most Y11 students have not used one before, so it is worth letting them have a go. Remind them to put the tape under the carbon paper and hammer! Equipment ticker timer, carbon and tape power supply and wires (if required) Sellotape or glue 37 Force and acceleration This experiment is likely to be done as a teacher demonstration for all but the most able classes. Equipment ticker-timer, tape and carbon power supply and wires (if required) trolley—check that it runs smoothly string pulley mass holder and masses books or blocks to tilt table slightly table (at least 1 m wide) Sellotape sheet of paper to stick tapes onto 38 The inclined plane—a simple machine This quick experiment shows the advantage provided by an inclined plane. Equipment force-meter small wood block of weight within the scale of the force-meter with hook or eye attached smooth flat wood or metal sheet (for the ramp) stack of books 39 Personal power By doing this experiment students get a feel for the value of a few kilograms and a watt. Equipment metre rule a balance that can weigh a stool (or weigh a lab stool using the gym scales) stopwatch or clock with a second hand 40 Heat capacity Practicals like this one have appeared in recent examination papers.... If you have sufficient samples it is worth students carrying this out themselves, rather than as a class demonstration. Equipment Styrofoam cups (available from supermarkets)—one per sample 100 mL measuring cylinders samples (lumps) of different substances, eg different metals (aluminium, copper, iron), other elements (silicon, sulfur), brick, china, rocks (sandstone, granite, basalt, greywacke). Each object must be small enough to be completely covered by water when placed in the Styrofoam cup thermometer large beaker Bunsen burners, tripod, gauze mat tongs or a fork to lower and lift objects 41 Phase changes of water Students should know the results of this experiment by now—but many need reminding. This practical also gives them practice at reading scales. Note: theory tells us the ice-water mix should remain at 0 °C until all the ice has melted, but it is more likely students will get results in the 1–3 °C range. Concentrate on the fact that the temperature remains constant, rather than the exact value (which is a function of air pressure and the accuracy of the thermometers). Similarly with boiling point. Equipment ice boiling tube thermometer beaker Bunsen, tripod and mat 42 Convection Part A This is best done as a demonstration over a large sink. Equipment gas jars potassium permanganate solution (or food dye or ink to colour water) hot and cold water thin card or plastic (overhead transparency acetate is good)—about 10 cm square Part B A small heat source is better for this than a Bunsen, since you want a local hot-spot in otherwise cold water. Equipment large beaker (600 mL or 1000 mL if possible) potassium permanganate crystal(s)—use one or two large crystals tweezers (potassium permanganate stains fingers) a dry milk-shake straw or glass tube (the tube from a broken pipette is good) heat source, eg candle or 12 V bulb. 43 Conduction Part A This is another common examination question. Students should concentrate on making accurate observations of what happens. Equipment rods of different substances eg aluminium, brass/copper, iron, glass Bunsen and tripod candle newspaper Part B Students are surprised to see water boiling at the top of the tube while the ice at the bottom stays solid. Equipment ice boiling tube steel wool test tube holders Bunsen 44 Experimental design: radiation Here is another chance to practise Fair Tests and writing down the obvious. This experiment would also be suitable for NCEA Science 1.1. Most students think that black surfaces heat up quickly and stay hot a long time. While this experiment can be done using cans (soft drink) that have been painted or polished, all you need are 100 mL glass flasks. Wrap one flask with aluminium foil, and paint the other black. If you have no black paint, mix a little PVA glue with charcoal. (The PVA 'paint' can be handled when dry without leaving marks on skin or clothes. If you want the flask back, it washes off easily in hot water and soap.) Note: if time is short, 20 minutes is usually enough to show the difference between the flasks. Equipment (Parts A and B) black and silver flasks stoppers containing thermometers to fit flasks heat source—an electric bar heater is good but a Bunsen will do box to elevate flasks in front of heater (optional) measuring cylinder kettle or Bunsen for boiling water A complete beginner's guide to attacking bacteria Antiseptic solutions: W = Water, D = Dettol, C = Cetrimide, L = Listerine, S = Savlon Antibiotics: W = Water, T = Tetracycline, P = Penicillin G, G = Gentamicin, C = Chloramphenicol The goal of this experiment is plates of a single bacterial culture with clear zones around the antibiotic and antiseptic solutions tested. Unless sterile techniques and equipment are used the plates can become contaminated with other colonies that will respond differently to the test solutions and confuse the results. For best results: • prepare the 1 cm2 filter paper squares (discs are even better), labelling them with pencil if desired, and then wrap in foil and bake for 3 hours at 120 °C • sterilise a bottle of water at the same time • use metal tweezers, soaking them in alcohol for a minute, then flaming them (ie burning off the alcohol) between each use. You need to completely cover each agar plate with the bacteria. This is best achieved by growing the bacteria in a nutrient broth (i.e. nutrient agar without the agar itself) for a day, then dipping a sterile swab (or cotton bud) into the broth and wiping that all over the agar. It is important that the bacteria in the broth be actively growing (i.e. fresh out of the incubator, not kept in the fridge for a week). • Prepare some sterile nutrient broth. • Sterilise a wire loop by heating it red hot in a flame, then allow it to cool. (You'll kill the bacteria if it is still hot.) • Use the sterile wire loop to take a little of the bacteria from a single colony off a cultured plate. • Open the bottle of broth and sterilise the neck by holding it over a flame and letting the flame enter the top of the bottle. • Put the loop of bacteria into the broth and swirl it to get the bacteria into the broth. • Reflame the bottle, then replace the lid. • Soak the wire loop in alcohol for a minute then flame it. • Label the bottle with the culture and date, then incubate the broth at the recommended temperature (about 30 °C for skin bacteria) for about 24 hours. • Check that the nutrient broth solution has turned cloudy. (If it is not cloudy the bacteria have not reproduced and you will have to either leave it for another day or try again with a fresh solution.) • To coat each plate with bacteria: • Open the nutrient broth and flame the neck as above. • Dip a sterile cotton swab into the broth, open the Petri dish, gently wipe (zig-zag) the agar with the swab, rotate the dish and wipe again in a different direction, rotate a third time and wipe again. (Do not dip the swab back into the broth between wipes.) You should just be able to see the zig-zag marks on the surface of the agar. • Replace the lid on the dish, discard the swab and repeat for the next plate. • All used swabs should be sterilised before disposal. To complete the experiment • Prepare the antiseptic solutions as per manufacturer's directions. • If you wish to investigate dilution effects, try using the solution at the stated concentration, then half-, quarte- and eighth-strength. • Either use filter paper labelled with pencil and record the code for each solution, or label the agar side of the Petri dish with a felt pen before placing each paper square. • Use sterile metal tweezers to dip the paper into each solution. Either blot the paper on a new tissue, or let it drip on the side of the beaker before placing it on the inoculated agar. The squares should be dipped but not dripping. • Each plate should also have a piece of paper dipped in sterile water. • Each experiment should be done in at least duplicate. Once each plate has been completed, seal it with tape and place it upside down. • Once all the plates are ready, place them upside down in the incubator at the appropriate temperature.