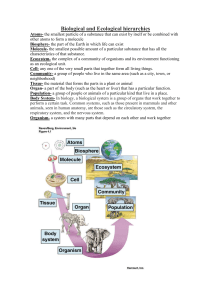
3 Ecological Indicators - National Water Commission
advertisement

Investigating Ecological Indicators of Stress from Water Scarcity A discussion paper prepared for the National Water Commission WAYNE ROBINSON numbersman.com.au MARCH 2010 i National Water Commission Cover Note This report is one of several reports prepared for the ‘Assessing Water Stress in Australian Catchments and Aquifers’ project (formerly titled the National Inventory of Water Stressed Catchments and Aquifers) which provides an assessment of the extent to which surface and groundwater systems have been hydrologically altered because of water extraction, regulation or flow alteration. Overarching report NWC, 2012, Assessing water stress in Australian catchments and aquifers, National Water Commission, Canberra. Supporting reports Marsh, N 2010, Hydrological indicators of water stress, report prepared for the Bureau of Meteorology for the National Water Commission. O’Keefe, V and Hamstead, M 2010, Understanding jurisdictional and Murray Darling Basin Authority approaches to identifying water stress, report prepared for the National Water Commission. Robinson, W 2010, Investigating ecological indicators of stress from water scarcity, discussion paper prepared for the National Water Commission. Sinclair Knight Merz, 2012, Assessing water stress in Australian catchments and aquifers, technical report prepared for the National Water Commission. Note - supporting reports do not necessarily reflect the views or opinions of the Commission. ii Executive summary The National Inventory of Water Stressed Catchments and Aquifers project proposes to develop an Australia-wide picture of water stress using a systematic and transparent approach that distinguishes where possible different causes of water stress, and is informed as much as possible by existing processes and indicators. This discussion paper forms part of that project and investigates the use of ecological indicators of water stress. Specifically, this paper investigates whether and how ecological indicators derived from existing data collections can be used to indicate the extent to which water systems across Australia have been impacted by surface and groundwater scarcity. The paper looks at in-stream biota (macroinvertebrates, fish, plants, algae, plankton and miscellaneous taxa), in-stream biological processes, riparian and floodplain vegetation and water birds as potential indicator groups. An extensive literature review of the relationships of these groups to water and flow regimes revealed many responses and tens of potential ecological indicators. The paper concludes that there are no ecological indicators that specifically respond to water-scarcity related stress. In other words, because of the complex nature of ecosystems and their response to stressors, none of the indicators trialled can report better on water scarcity than direct measurement itself. There are very few existing programs collecting ecological data in an unbiased manner that could be used to inform the national inventory. The candidate indicator with the most potential is Normalised Differential Vegetation Index (NDVI), generated from remote sensed reflectance values from riparian and flood plain vegetation. iii Table of Contents Executive summary................................................................................................ iii Table of Contents ................................................................................................... iv 1 Background ..................................................................................................... 1 1.1 1.2 1.3 1.4 Approach ................................................................................................... 2 End Uses ................................................................................................... 2 Definition and Scope .................................................................................. 3 Ecological Indicators Discussion Paper...................................................... 3 2 Introduction to Ecological Monitoring ........................................................... 4 3 Ecological Indicators....................................................................................... 5 3.1 What is an Ecological Indicator? ................................................................ 5 3.2 Indicator Development ............................................................................... 5 3.3 Indicator Variability..................................................................................... 6 3.4 Indicator Demonstration ............................................................................. 7 3.5 How could ecological indicators be used to indicate water stress in catchments and aquifers across Australia? ........................................................... 7 3.6 Standardising Indicators ............................................................................ 9 3.7 Indicator Responsiveness ........................................................................ 10 3.8 Which Indicators? .................................................................................... 11 3.9 Regional indicators .................................................................................. 11 3.10 It’s all a matter of scale ............................................................................ 11 3.11 Indicators Summary ................................................................................. 12 4 Identifying Potential Ecological Indicators of Water Scarcity for the Australian Environment. ....................................................................................... 13 4.1 4.2 4.3 4.4 4.5 Literature demonstrating the use of ecological indicators of water scarcity 13 In-stream biota ......................................................................................... 13 Waterbirds ............................................................................................... 20 Vegetation ............................................................................................... 23 Biological Processes ................................................................................ 26 5 Potential suitable indicators for ecological indicators of water scarcity from responses cited in the literature.................................................................. 29 5.1 5.2 5.3 5.4 5.5 5.6 5.7 6 Macroinvertebrates .................................................................................. 29 Fish .......................................................................................................... 30 Algae/Plankton......................................................................................... 31 Miscellaneous biota ................................................................................. 31 Waterbirds ............................................................................................... 32 Vegetation ............................................................................................... 33 Biological processes ................................................................................ 34 Current Status of Ecological Indicators ....................................................... 34 6.1 6.2 6.3 6.4 In-stream biota ......................................................................................... 34 Waterbirds ............................................................................................... 37 Vegetation ............................................................................................... 39 Biological Processes ................................................................................ 41 iv 7 Identification of indicators feasible to use, based on criteria including whether adequate data exist to populate the indicator. ..................................... 41 7.1 7.2 7.3 7.4 7.5 8 Introduction .............................................................................................. 41 In-stream Biota ........................................................................................ 41 Waterbirds ............................................................................................... 42 Vegetation ............................................................................................... 43 Biological Processes ................................................................................ 43 Options and recommendations for the way forward................................... 44 8.1 Introduction .............................................................................................. 44 8.2 In-stream Biota ........................................................................................ 44 8.3 Waterbirds ............................................................................................... 48 8.4 Vegetation ............................................................................................... 48 8.5 The way forward using NDVI ................................................................... 49 Before using NDVI ............................................................................................... 53 9 Alternative view ............................................................................................. 54 10 Conclusion ..................................................................................................... 55 Appendix 1 Water birds literature review ............................................................ 56 A1.1 Literature Review of Waterbird Ecology relevant to water use and water scarcity ................................................................................................................ 56 A1.2 There’s lots we need to consider before using waterbirds as indicators ... 63 A1.3 Literature Review of Waterbirds as indicators .......................................... 66 Appendix 2 Literature Review of Vegetation Ecology relevant to water scarcity ................................................................................................................................ 77 Appendix 2.1 README FILE FOR MODIS-DERIVED Land Products (Paget and King 2008) ........................................................................................................... 92 Appendix 3 Summary of literature review of in-stream biota ecology relevant to water scarcity ..................................................................................... 98 Acknowledgements............................................................................................. 106 References ........................................................................................................... 107 v 1 Background One of the key aims of improved water planning and management under the National Water Initiative (NWI) is to avoid over-allocation and overuse of surface and groundwater systems, and to return already over-allocated and/or overused systems to sustainable levels of extraction. The National Water Commission’s (NWC) 2009 and 2007 Biennial Assessments on progress of water reform have identified shortcomings in how water plans implement the concept of environmental sustainability and in the recovery of water from over-allocated and overused systems. Consequently, the NWC has identified addressing over-allocation and overuse in water systems as a high priority. This is reflected in our Sustainable Water Management Improvement Strategy and NWC Water Dependent Ecosystem Position Statement. A major difficulty in addressing over-allocation and overuse issues is the absence of a nationally agreed method for determining the sustainable levels of extraction within a water system. As a consequence the extent of overallocation is unknown at a national level. However, in the absence of an agreed procedure, the Commission believes it is possible to provide defensible evidence of the extent to which water scarcity is causing significant water stress to water systems. This scarcity can arise from climatic changes and other causes as well as from water extraction and use. The National Inventory of Water Stressed Catchments and Aquifers project proposes to develop an Australia-wide picture of water stress using a systematic and transparent approach that distinguishes where possible between different causes of water stress, and is informed as much as possible by existing processes and indicators. 1 1.1 Approach The Inventory is being developed through the following stages: (a) Understanding existing approaches to identifying water stressed catchments and aquifers, e.g. those used by jurisdictions, the MBDA and the CSIRO Sustainable Yields work (b) Producing a series of maps, and an associated report, of the hydrological stressors or pressures present in each catchment/aquifer (to be undertaken by the Bureau of Meteorology in collaboration with CSIRO and other specialists) (c) Clarifying the feasibility of using ecological indicators that are sensitive to water scarcity to help determine levels of water stress via a discussion paper and workshop (this project). If the approach is feasible, an ecological indicators project will be implemented (d) Commissioning discussion papers related to other dimensions of water stress and, if feasible and supported by the Steering Committee, developing indicators (e) Integrating and packaging the above to produce a national inventory of water stressed catchments and aquifers, and supporting documents. 1.2 End Uses The inventory is intended for national use. The inventory will assist the National Water Commission in: Undertaking periodic national assessments such as Biennial Assessments Encouraging nationally consistent interpretations of water stress and building public understanding of the extent of water stress Informing the priorities, processes and programs of the NWC and other organizations Achieving aspects of the National Water Initiative relevant to its charter. 2 1.3 Definition and Scope For the purposes of this inventory, water stressed catchments and aquifers are those that demonstrate signs of significant surface and/or ground water scarcity as indicated by: Available jurisdictional assessments of water scarcity or water stress The hydrological stressors present in the catchment/aquifer relevant to ecological processes and, depending on the results of scoping investigations, o The state of the water scarcity-sensitive ecology in the catchment/aquifer area o The understanding of communities in the catchment/aquifer area about the impacts of water scarcity on that aquatic environment. Relative levels of water stress will be identified. How the levels will be categorised, and whether the above indicator ‘layers’ can be combined into a single index of extraction-related water stress, will be examined as the project progresses. Where possible, climate induced stress will be distinguished from extraction induced stress. The inventory is focusing on stress related to water scarcity, not too much water. 1.4 Ecological Indicators Discussion Paper This project will investigate whether and how ecological indicators derived from existing data collections can be used to indicate the extent to which water systems across Australia have been impacted by surface and groundwater scarcity. Chapter 2 introduces ecological monitoring, Chapter 3 defines ecological indicators, Chapter 4 summarizes the responses of the ecological groups to changes in water regimes from a thorough literature review, Chapter 5 looks at the potential indicators that come through that literature review and speculates as to what sort of data would be required, Chapter 6 looks at the available data from existing monitoring programs, Chapter 7 summarizes the preceding three chapters into feasible indicators for each ecological group and Chapter 8 looks at the best way forward for all groups. 3 2 Introduction to Ecological Monitoring Freshwater organisms have been used widely within Australia and globally for environmental monitoring (e.g. Barbour et al. 1992; Norris et al. 2007b). These bioindicators are used to assess responses to environmental stresses (e.g. organic pollution), particularly physico-chemical characteristics affected by landuse (e.g. agriculture, urbanisation or mining)(e.g. Barbour et al. 1992; Chessman 1995; Johnson et al. 2006). Biological monitoring in freshwater systems has been undertaken using a range of taxa, including invertebrates, algae, fish, plankton, plants and terrestrial fauna (e.g. birds) – with indices based on individual groups and/or combinations. Recent examples of studies that report on assessments of freshwater systems using several groups of organisms include O'Connor et al. 2000 (invertebrates, diatoms, zooplankton, fish and birds); Johnson et al. 2006 (fish, macrophytes, benthic diatoms and macroinvertebrates); and (Angradi et al. 2009)(fish, macroinvertebrates, phytoplankton, zooplankton, aquatic vegetation). The term ecological indicators can refer to ecological components (e.g. soils, biota, physical form, etc), ecological processes (e.g. climate, nutrient dynamics, species interactions, etc.) or ecosystem services (e.g. maintenance of hydrological regime, recreation and tourism, educational, etc.) (Department of the Environment Water Heritage and the Arts 2008). In this discussion paper I stick to where the bulk of the literature, research and jurisdictional programs are aimed—at the ecological components of aquatic biota and associated processes. I concentrate on five main groups of assumed importance from my experience with existing monitoring programs. The themes chosen are: vegetation (wetland and riparian), macroinvertebrates, fish, waterbirds, and algae and plankton. The in-stream components of macroinvertebrates—fish, algae, and plankton—are reviewed together under the umbrella of freshwater biota, whilst waterbirds and vegetation stand alone. Because of the complex nature of ecosystems their function can be quantified using measures of ecosystem process (Bunn et al. 1999) and I have included this as well. There is no relevant ecological monitoring of groundwater dependent ecosystems and these are unfortunately only briefly addressed. 4 3 Ecological Indicators Sections 3.1 to 3.4 and Section 3.7 are adapted from the United States Environment Protection Agency’s Environmental Monitoring Assessment Program, Research Strategy (McDonald et al. 2002). 3.1 What is an Ecological Indicator? An indicator is one (or more) measure(s) or model(s) that describes the condition of the system in question (e.g. blood pressure is an indicator of human health, an ecological indicator is an indication of ecological health). Effective aquatic ecological indicators are central to determining the condition of aquatic resources. The most successful ecological indicators have been multi-metric indices formed by combining biological indicators within a taxon (e.g. those indices related to fishes—number of species present, number of pollution-tolerant species, etc.) found in aquatic ecosystems. Separate indices for different taxa (from plants, birds, vegetation, fishes, etc.) are important because they may be critical components of aquatic ecosystems, and because they integrate various natural and anthropogenic stressors into their responses. Indicators can stand alone or as flags that trigger additional measurements of the fundamental and associated components of the abiotic environment, including physical measures of habitat (e.g. substrate type and quality, depth) and chemical measures of the ambient water quality (e.g. dissolved oxygen, temperature, salinity, nutrients, and toxics). 3.2 Indicator Development Indicators can be developed for any level of biological organization (Table 3.1). However, the structural and functional aspects of the biological or ecological characteristic to be used as an indicator must be appropriate to the question being asked. 5 Table 3.1. Levels of biological organization to consider during indicator development with examples of structural and functional aspects of each level (Adapted from McDonald et al. 2002). Structure Level of Organization Processes Heterozygosity Gene Polyploidy Rate Mutation Rate Recombination Rate Condition Anomalies/Deformities Maximum Size Tissue Contamination Individual Metabolic Rate Growth Rate Fecundity Abundance Age Class Distribution Size Class Distribution Population Reproduction Rate Growth Rate (of Population) Death Rate Evolution/Speciation Relative Abundance Richness -Native Richness - Total Evenness Trophic Composition Reproductive Composition Habitat Guilds Assemblage (Community) Competition/Predation Disease/Parasitism Mutualism Recovery Rate Regional Diversity (gamma) Homogeneity Hot Spots Patches Patterns Fragmentation/Recovery Watershed or Landscape (Basin or Catchment) Water Delivery Chemical Delivery (Native and Exotic) Material Delivery (Sediment, Wood) Energy Flow Nutrient Cycles and Spiraling Population Sources and Sinks Fragmentation Rate/Recovery Rate 3.3 Indicator Variability An often overlooked, yet essential component of any monitoring program is the description of the components of variability, which impacts status and trends in the indicator. The important consideration is the extent to which variability in measuring the indicator (noise) masks detection of significant changes. This may be natural variability such as the real differences within a body of water or real differences from the period when the sampling site is visited. It also contains variability created by the samplers and differences among samplers, the laboratory/data processing and other extraneous components. Ultimately, there is little point in implementing an indicator on 6 conceptual relevance alone. Sufficient information on indicator variability is needed to determine the power of the indicator to detect a change or trend. In snapshot studies, the indicator must be able to determine the status of the ecosystem to within acceptable levels of tolerance. 3.4 Indicator Demonstration During the final stages of indicator development, potential users must be given some sense of how well the indicator performs and how it can be used. This often implies demonstrating the indicators in a regional scale project, showing the results and potential conclusions, which may derive from using the indicator. This is part of a necessary “proof-of-concept” for the indicator to be accepted into widespread use. Indicator demonstration would be the next step in this project if potential indicators are found and recommended. 3.5 How could ecological indicators be used to indicate water stress in catchments and aquifers across Australia? The concept is that ecological indicators in ecosystems that are subjected to stress from water scarcity will be detectably different from those in unstressed systems. In the simplest scenario for this particular project, the ecosystem would only be subjected to stress from water scarcity and any changes in the indicator (from some predetermined ‘reference’ or ‘healthy’ value) would be attributable to water scarcity related stress (Figure 3.1). Even if this type of ecosystem could be found it would be in the minority as the majority of Australian aquatic ecosystems are exposed to multiple stressors. To deal with ecosystems exposed to multiple stressors, including water scarcity, it is desirable to find indicators that either a) react only to water scarcity, or b) can partition water scarcity effects from other stressor effects. An example of these scenarios is shown in Figure 3.2. One of the stressors that may already be affecting and could affect all ecosystems in the future is climate change. 7 Changed Ecological indicators Ecological indicators Healthy Ecosystem functioning normally Water scarcity Stressed Ecosystem Figure 3.1: Concept of using ecological indicators to identify stressed ecosystems in the ideal situation where water scarcity is the only stressor affecting the indicators. 3.5.1 Dealing with climate change and other stressors The effects of other stressors in the system, such as Climate Change, are easily dealt with in a statistical sense by using reference sites. Whether or not climate change effects in general should affect water allocations is independent of this paper. Statistically speaking, to partition out the effects of climates change, reference/comparison sites/units can be used. For example; Assume or locate several sites that have the same exposure to climate change (e.g. similar geography and climatic histories) One or more of the sites are known not to be exposed to water scarcity related stress during the monitoring period and are treated as reference sites Changes in the chosen indicator(s) in the other sites are compared to the (non-water stress related) changes in the reference sites and an evaluation made. This method works best if the reference and assessment sites are a similar as possible in as many ways as possible before the assessment period, but it is 8 not essential. Finding more than one reference sites may help considerably. This method is akin to the example in Figure 3.2a. a) Ecological indicators Healthy Ecosystem functioning normally Changed land use, Climate change, Chemical pollution, etc etc. Including water scarcity Multiple stressors Changed Ecological indicators Stressed Ecosystem b) Changed land use, Climate change, Chemical pollution, etc etc. Multiple stressors Ecological indicators Already Stressed Ecosystem Changed Ecological indicators Water scarcity Stressed Ecosystem Figure 3.2: Concept of using ecological indicators to identify stressed ecosystems where multiple stressors affect the ecological indicators. a) A component of change in the indicator can be attributed to water scarcity, and b) the ecological indicators are designed or adjusted to react only to water scarcity. 3.6 Standardising Indicators Indicators need to be standardised for comparison. The standardising can be in the sampling protocol or calculation of the indicator, or both. For example, 9 the Sustainable Rivers Audit (SRA) uses a standardised sampling protocol for all fish sampling in the MDB. This makes it possible to directly compare across sites or regions in, say, number of fish or number of fish species or number of herbivorous fish species, etc. On the other hand, the macroinvertebrate sampling has slight differences between jurisdictions (live pick Vs lab sort). But the comparisons are made possible because the number of species is compared against a predicted number of species or community structure for the relevant sampling method. The raw data are rarely used for reporting purposes and the indicators are typically called ‘Indices’ or ‘Metrics’. An index is the score after standardising for sampling. For example, an AUSRIVAS OE score is the Observed to Expected Ratio and although more extreme values can occur; for macroinvertebrates this typically takes a value between 0.3 and 1.5. In the Sustainable Rivers Audit the term metric refers to an index that has generally been range standardised to only take values between 0 and 1. When referring to indicators, this paper talks about the variable measured and when referring to metrics I infer the SRA definition above; i.e. the indicator called species richness is the number of species in the taxon of interest, the metric called species richness is a ratio of how fit the spatial unit (site or region) is, based on species richness in that group. 3.7 Indicator Responsiveness Evaluating the degree to which a particular indicator actually responds to stressor gradients is an important aspect of the indicator development process. Without some knowledge of the shape of the response curve and the variability associated with the response, it is difficult to evaluate the utility of any particular indicator. For example, an indicator such as percent native vegetation can only take a value between 0 and 1, but this does not make it a metric. Proportions are non-linear, particularly near the extremes and so a score of say 0.99 is not the same distance from 0.98 as a score of 0.51 is from 0.50. Understanding the responsiveness is part of the process used to generate the metrics in monitoring programs like the SRA. 10 3.8 Which Indicators? One of the requirements of this discussion paper is to review potential ecological indicators sensitive to water scarcity that could be used to indicate water stress either across Australia, or in parts of Australia. Preference is to be given to using existing programs and data where possible. Several approaches could be taken on this. One approach would be to review existing programs first, then determine whether there is anything relevant to water scarcity being collected. The second approach would be to determine what relevant indicators are needed and then to interrogate existing data and programs to find compatibilities. The former approach is certainly more time efficient but risks not finding as many potential indicators. Because no jurisdiction currently uses ecological indicators of water related stress per se, the second approach should locate many more relevant potential indicators. Many of these potential indicators will not be available ‘off the shelf’, but the approach has a higher chance of finding a way forward. Therefore the approach I have taken is the latter of the two. I start by reviewing the literature and experts in detail and then drill into the available data to search for matching data and indicators. 3.9 Regional indicators As is noted for river health indicators in the Framework for the Assessment of River and Wetland Health (FARWH) (Norris et al. 2007a) it is quite reasonable to have different indicators for different regions. In fact it would be expected that this were the case because the ecological responses of different ecosystems must be related to the organisms and processes naturally occurring there. For example, floodplain vegetation may be an ideal indicator for floodplain ecosystems but it is totally irrelevant in regions where floodplains do not occur. 3.10 It’s all a matter of scale Scale is one of the most important issues for this project. Scale of response for each indicator and scale of reporting required for the project are very different matters. For example, it may be found that wilting in individual trees 11 is a very good indicator of water scarcity related stress. How to turn this into an indicator is one question and how to report tree wilting on a national scale is another. Each potential indicator will need to address a response scale and how it may be used in a national reporting framework. Temporal scale also plays a huge role in this project. How long does the response unit have to be exposed to scarce water before it responds? Generally speaking the aggregating of indicators to larger spatial scales has been well studied and indicators developed in this report can be aggregated using the FARWH protocol (Norris et al. 2007a) 3.11 Indicators Summary Indicators of any kind need to be responsive, have known relationships and be suitable for the question(s) being asked. This report will list currently used ecological indicators in Australian aquatic resource monitoring programs and propose more, based on conceptual relationships using the literature review and expert opinion. However, any proposed indicators and not even all those currently in use have been subjected to rigorous evaluation of sources of variability and ultimately, ecological sensitivity. That is beyond the scope of this discussion paper but recommendations will address a way forward. 12 4 Identifying Potential Ecological Indicators of Water Scarcity for the Australian Environment. Considerable literature reviews were performed for this section but only summaries are presented here. The literature reviews in detail are provided as appendices. 4.1 Literature demonstrating the use of ecological indicators of water scarcity There is very little existing literature looking at water scarcity and ecological relationships. However, there are a number of studies (mostly Australian) reporting on closely related topics such as the development of ecological indicators for use in monitoring aspects of flow alteration. The potential of a range of organisms to be used as indicators of the effects of environmental water allocations on wetlands of the Murray–Darling Basin were discussed in Reid and Brooks (2000). In a similar approach as outlined for this paper, their assessment was based on current knowledge of responses of particular taxonomic groups to physical and chemical changes associated with changes in water levels in wetland systems (Reid and Brooks 2000). 4.2 In-stream biota This section summarizes the literature relevant to macroinvertebrates, fish, algae and plankton. Benthic macroinvertebrates are one of the most common groups used for assessing ecological river quality. Benthic macroinvertebrates are capable of reflecting different anthropogenic perturbations through changes in structure or function in the assemblages and thus enable an overall assessment of streams (Hering et al. 2004). Examples of freshwater monitoring programs based on benthic macroinvertebrates include AUSRIVAS, which has been used in all states and territories throughout Australia (Norris et al. 2001) and AQEM (developed under the EU Water Framework Directive (Hering et al. 2004)). Sheldon and Thoms (2006) used an analytical approach to investigate relationships between macroinvertebrate assemblages and flow variability indices (including indices reflecting stream 13 permanence and connectivity). Similarly, the Lotic-invertebrate Index for Flow Evaluation (LIFE), for assessing the impact of variable flows on benthic macroinvertebrate populations (see also (Monk et al. 2006)), reported on a wide-scale application of LIFE across England and Wales (Extence et al. 1999). The LIFE approach is discussed in more detail in Appendix 3. Other studies investigated the suitability of using AUSRIVAS monitoring to assess the impact of dams (Marchant and Hehir 2002) and during periods of drought (Rose et al. 2008). (Growns 2008) assessed relationships between fish assemblages and an index of hydrological alteration in regulated rivers. Fish are also regarded as good bioindicators and are often used for the assessment of the ecological integrity of rivers, because of their longevity, mobility and sensitivity to habitat modification (e.g. Lasne et al. 2007; Oberdorff et al. 2002; Welcomme et al. 2006b). The more widely used freshwater bioindicators are based on community-level data (e.g. species richness, and diversity). This is the case for several macroinvertebrate indices, such as SIGNAL (Chessman 1995), AUSRIVAS (Davies 2000), family richness, EPT (Ephemeroptera, Plecoptera and Trichoptera) and OCH (Odonata, Coleoptera and Hemiptera) (Barbour et al. 1992). Fish based monitoring programs tend to include community, population and individual based metrics (e.g. Harris and Gehrke 1996). Algae and plankton have rarely been used as bioindicators in ongoing monitoring programs. 4.2.1 Relationships between hydrology and ecology of in-stream biota A conceptual diagram summarising effects of reduced flow on invertebrates is shown in Figure 4.1. 14 Figure 4.1: Summary of the effects of decreased stream flow on habitat conditions and invertebrate community abundance, diversity and composition (from Dewson et al. 2007). The general structure can be applied to other in-stream biota including fish, algae and plankton. 4.2.2 Responses of in-stream biota to water scarcity (i.e. reduced flow or drought) The most frequently reported responses to water scarcity (i.e. reduced flow/discharge or drought) for macroinvertebrates were decreases in diversity and abundance (Table 4.1). Changes to community composition were also reported by a number of studies. In particular, riffle habitat specialists tended to be replaced by pool-preferring taxa, as flow or discharge decreased. Several studies reported changes in patterns of drift (increases and decreases) in response to reduced flows (Table 4.1). 15 Table 4.1: Responses of macroinvertebrates to reduced flow or discharge, including drought. Flow characteristics affected Reduced flow (from drought and/or water diversion and/or abstraction) Biological or Ecological Response References Of the 22 studies cited in an earlier literature review, the most reported decreases in density (12 studies) and taxonomic richness (9 studies); and altered species composition (20 studies). Of the 11 studies cited that investigated drift, 10 reported increases. (Dewson et al. 2007) (review paper) Reduced community diversity; Increased number of pollution-tolerant, lentic taxa present in edge habitat; increased number of pollution-sensitive “pool-preferring” taxa in riffle habitat; reduced abundance of some taxa; altered community structure. (Attrill et al. 1996; Cowx et al. 1984; Rose et al. 2008; Suren and Jowett 2006) Increased drift for some species during (experimental) periods of reduced flow; altered species composition of drift invertebrates. (James et al. 2009; Tonkin et al. 2009) Changes to predation and competition (increases and decreases reported). (Malmqvist and Sackmann 1996; Matczak and Mackay 1990; Zhang et al. 1998) Reduced connectivity (cessation of flow/river reduced to fragmented pools or flow in the hyporheic zone only) Reduced diversity and abundance of rheophilous taxa (e.g. riffle-dwelling mussels, hydropsychic caddisflies); reduced recolonisation as a result of reduced drift; altered community structure (Boulton 2003; Golladay et al. 2004; Hose et al. 2005) Drought and/or water diversion or abstraction coupled with increased salinity and poor water quality Altered species assemblage (increased dominance of salinity-tolerant taxa) (Lind et al. 2006) Reduced frequency, duration and area of inundation of floodplain/wetlands Reduced abundance and biomass of invertebrates (Boulton and Lloyd 1992) Research concerning the responses of fish to water scarcity has involved many studies focused at population-scale monitoring (c.f. macroinvertebrate studies, which were mostly community-level), and the response most often reported was population decline (Table 4.2). Other responses detected in population-level studies included reduced growth rates and recruitment. Several authors also reported changes at the community scale and these included reduced diversity and/or increased prevalence of exotic species or habitat generalists, and decreased prevalence of riffle specialists (Table 4.2). 16 Table 4.2: Responses of fish to reduced flow or discharge, including drought. Flow characteristics affected Reduced flow (from drought and/or water diversion and/or abstraction) Biological or Ecological Response References Of the 50 studies cited in an earlier review, most reported population declines during drought (38 studies). Other prevalent findings were death attributed to poor water quality, crowding, decreased reproduction, altered community assemblages, altered movement patterns (increased movement during early stages of drought; decreased movement in later stages because of lost connectivity). (Matthews and MarshMatthews 2003) (review paper) Reduced abundance and biomass (overall); reduced richness and abundance of rifflespecialist species; some pool-dwelling species found to be insensitive to moderate flow reductions (e.g. 75-90% reduction of summer flow); reduced growth rates; altered community structure (increased prevalence of exotic species and/or habitat generalists); reduced Index of Biotic Integrity scores, increased competition and predation; reduced recruitment and/or failure to spawn. (Bradford and Heinonen 2008; Cowx et al. 1984; Freeman and Marcinek 2006; Harris 1988; Magoulick and Kobza 2003; Marchetti and Moyle 2001; Osmundson et al. 2002; Osmunson et al. 2002) Long-term flow reduction Population declines and local extinctions; reduced growth rates and recruitment for some species. (Bond et al. 2008; Matthews and MarshMatthews 2003) Reduced connectivity (cessation of flow/river reduced to fragmented pools or flow in the hyporheic zone only) Reduced diversity and abundance of rifflespecialist species; crowding (i.e. fish trapped in dry season refuges with deteriorating physicochemical conditions and reduced food availability); increased competition and predation, increased rates of disease, parasitism and mortality; stranding of fish (particularly larvae and juveniles); altered community structure. (Bradford 1997; Bradford et al. 1995; Freeman et al. 2001; Kushlan 1976; Lowe-McConnell 1985; Magoulick and Kobza 2003; Medeiros and Maltchik 1999; Pusey et al. 2004; Woodland and Ward 1990) Reduced frequency, duration and area of inundation of floodplain/wetlands Reduced spawning areas and/or recruitment success of lowland river fish. (Cadwallader and Lawrence 1990; Geddes and Puckridge 1989; Jubb 1972; Lake 1975; Welcomme 1979; Whitley and Campbell 1974) The responses of fish need to be considered on a site-by-site or case-by-case basis. For example, a recent study found that a hydrological index that described hydrological change in six regulated rivers in the Murray–Darling Basin explained only a small amount of variation in fish assemblage structure and the abundances of individual fish species (Growns 2008). This may reflect the general nature of the hydrology index used, a composite measure of various aspects of hydrological change. 17 In comparison to macroinvertebrates and fish, literature documenting the responses of algae and plankton to water scarcity was limited (Table 4.3). Of the few studies identified, most reported increases in algal biomass in response to reduced flow or drought, but decreases in diversity and altered community structure were also documented. Table 4.3: Responses of algae and plankton to reduced flow or discharge, including drought. Flow characteristics affected Reduced flow (from drought and/or water diversion and/or abstraction) Drought and/or water diversion or abstraction coupled with increased salinity and poor water quality Biological or Ecological Response References Of the 7 studies cited in an earlier literature review, most reported increases in algae (6 studies); some reported mixed responses. (Dewson et al. 2007) (review paper) Reduced production and altered community assemblage of benthic algae (and substantially so, if flow reduction results in the loss of permanent pools) (Robson and Matthews 2004) Altered periphyton biomass. (Tonkin et al. 2009) Increased production of phytoplankton associated with flow reduction (Lloyd et al. 2003) Changes in species composition of zooplankton (Nielsen et al. 2005; Zhou et al. 2008) Decreased species diversity and richness of diatoms in streams with increased secondary salinity (attributable to heavy irrigation and dryland farming) (Blinn and Bailey 2001) Examples of the responses of other freshwater organisms to water scarcity were also found in the literature (Table 4.4). Several population-level effects were documented for shrimp, including reduced reproductive success, reduced migration of larvae and altered size-class distribution. Drought was found to be associated with decreased richness but increased abundance of emergent propagules (invertebrates, protista and algae). Lastly, related research on two turtle species demonstrated that reduced flows were likely to result in decreased reproductive output (pig-nosed turtle), and drought (pond drying) resulted in an increase in emigration rates and stress levels in slider turtles. 18 Table 4.4: Responses of other freshwater organisms to reduced flow or discharge, including drought. Organism Flow characteristics affected Biological or Ecological Response References Shrimp Reduced flow (from drought and/or water diversion and/or abstraction) Reduced migration of shrimp larvae; Increased densities in shallower and smaller pools; increased competition for resources and decreased reproductive success; altered sizeclass distribution. (Covich et al. 2003; Pringle and Scatena 1999) Wetland sediment propagules (invertebrates, protista and algae) Drought and/or water diversion or abstraction coupled with increased salinity and poor water quality Decreased richness and increased abundance of emergent propagules associated with (experimentally) increased salinity (i.e. similar to conditions that may be present as a result of hydraulic alteration in natural wetland systems) (Skinner et al. 2001) Turtles Reduced flow (modelled water abstraction) Reproductive output of pig-nosed turtle predicted to decrease (based on models of reduced river flows), as a result of habitat fragmentation, reduced access to food and nesting resources. (Georges et al. 2003) Pond drying (drought) Slider turtle (not an Australian endemic) showed behavioural (increased emigration) and endocrine (increased levels of “stress” hormone) responses to decline in habitat quality (pond drying). (Cash and Holberton 2005) 19 4.3 Waterbirds 4.3.1 Relationships between hydrology and ecology of waterbirds Waterbirds were approached vigorously because there are several features of their ecology that appealed to make them potentially suitable candidates to supply indicators. They are widespread and by definition they are water dependent birds. An interesting feature of their ecology is that they are at the top of a chain of events related to water dependency. That is, they are dependent on water itself, but also reliant on other ecosystem responses to water or the hydrological regime. For example, they rely on suitable nesting habitat, which may be floating macrophytes, which in turn are reliant on the hydrology of the system. Or they may be dependent on eating the fish, which may be dependent on eating the macroinvertebrates, which may be dependent on grazing on the algae, which may be dependent on the nutrients, which may be dependent on the soil, which may be dependent on the hydrology. There are many, many such complex interactions between waterbirds and their environment and unfortunately this means that there are many, many ways that they may become absent from, or less productive in an area. In other words, there are no immediately perceivable indicators of water scarcity for waterbirds that aren’t confounded by at least one other variable. Even persistence or presence at site is a poor indicator, because they can move quite readily and absence doesn’t mean poor condition (Kushlan 1993). One unique feature of this group is their capability for migrating across catchments. This has two consequences: their absence may tell us nothing because they just happen to be somewhere else; or their presence means that at least the area they are in can support them. There is a lot of emphasis in the literature on breeding events in waterbirds, but this is difficult to interpret as a potential indicator because of the inherently variable nature of breeding and their migration patterns. For example, breeding only once in 8 years will sustain the populations of some species in some areas(Lesley 2001). In years when they aren’t breeding in an area they may be doing so somewhere else. So in the long run if the species persists at the regional or national scale, then it will probably migrate to other areas when opportunities arise. So how can a breeding indicator be used? Perhaps one could say that in region 20 X (say, Hattah Lakes) if species Y has bred and fledged young twice in the last 10 years, then that is normal. Well, if that become an indicator then at least 10 years of data would be required for reporting. Besides which the sensitivity is questionable because statistically speaking it would not be uncommon for a 1 in 5 year flood to occur only once or not occur at all in a 10-year period. So non-breeding may tell us there is a potential water shortage in that area, but not whether that is an unnatural shortage. However, if there were long-term data that showed that species Y has never gone more than, say, 5 years without breeding before, then there is a way forward. Hence a problem with many potential indicators in most themes, but waterbirds in particular, is the need to partition natural variability. Because they are an icon group of species with reasonably high public appeal, the waterbirds literature review (Appendix A1) is comprehensive and many options are explored. Waterbirds have recently been adopted in some monitoring programs (see Chapter 7) albeit with little research into sensitivity. 21 4.3.2 Responses of waterbirds to water scarcity (i.e. reduced flow or drought) There are no papers looking at water scarcity per se. A summary of known responses to hydrological alterations is given in Table 4.3.1. Most literature is relevant to wetlands or floodplains and little refers to the river channel. Table 4.3.1: Responses of waterbirds to hydrological alteration. Flow characteristics affected Reduced Wetlands Area Biological or Ecological Response References Decreased Waterbird Abundance (Kingsford and Thomas 2004), (Lesley 2001), (Kingsford et al. 1999) Decreased Waterbird Diversity (Green et al. 2002) Reduced Breeding events (Hughes 2003; Kingsford and Johnson 1998), (Eglington et al. 2008), (Kingsford and Norman 2002) Reduced Breeding Success (Russell et al. 2002), (Kushlan 1993) Increased migration (Kingsford and Norman 2002), (Kingsford et al. 1999) Decreased waterbird population (Kingsford and Johnson 1998) Change in diet (Kingsford and Norman 2002) Increased waterbird abundance (Kingsford et al. 1999), (Dorfman et al. 2001) Increased waterbird breeding (Lesley 2001) Increased waterbird abundance (Bellio et al. 2009) Decreased Breeding success (Desgranges et al. 2006) Fewer breeding events (Lesley 2001) Changed community composition (Kingsford and Thomas 2004) Decreased Flood magnitude Decreased breeding (Kingsford and Auld 2005) Changed timing of flood Decreased breeding (Lesley 2001), (Kingsford et al. 1999), (Kingsford and Norman 2002) Permanence of flow Decreased waterbird diversity (Kushlan 1993), (Robledano et al. 2009) Reduced Flow Volume Wetland drying phase Variability in water depth Decreased Flood Frequency 22 4.4 Vegetation 4.4.1 Relationships between hydrology and ecology of vegetation There is little literature on in-channel vegetation and this section refers to riparian and floodplain vegetation in the main. Vegetation requires water and differs from the other themes in that it potentially uses surface water, ground water and rainfall, hence is a potentially better indicator of overall water stress. Literature from the theme is really dominated by contemporary studies and there are significant keystone papers in the last few years. Intuitively there is a lot of potential in this theme because of a couple of significant biological traits unique to vegetation: 1) Individuals don’t move, if there is a water scarcity issue, the tree(s) must weather it out, they can’t move. 2) The known responses to stressors can apply and be measured at the individual or population (e.g. stand of trees) scale. This theme threw up considerably more potential indicators than the others and certainly has fewer issues with confounding than any of the others. In other words, whilst there are other potential stressors to vegetation, many effects can be readily differentiated from water stress effects. 4.4.2 Responses of vegetation to water scarcity (i.e. reduced flow or drought) Known and potential responses were well summarised by (Poff and Zimmerman 2010) but in Table 4.4.1 I have included many others as turned up in the literature review (Appendix A2). One finding from the literature review is that there is a tendency to monitor overall vegetation health, as well as or instead of simply responding to individual stressors, so I have also included a table listing recommended general indicators of vegetation health from a couple of key contemporary papers (Table 4.4.2). 23 Table 4.4.1: Responses of vegetation to reduced flow or discharge, including drought. Flow characteristics affected Lower peak flows Biological or Ecological Response References Change in recruitment, failure of seedlings (Poff and Zimmerman 2010) Terrestrialisation of flora (Poff and Zimmerman 2010) Increased exotic success (Poff and Zimmerman 2010) Lower species richness (Poff and Zimmerman 2010) Vegetation encroachment (Poff and Zimmerman 2010) Increased Riparian cover (Poff and Zimmerman 2010) Change in community composition (Poff and Zimmerman 2010) Decreased species richness (Poff and Zimmerman 2010) Increased wood production (Poff and Zimmerman 2010) Reduced growth rate (Poff and Zimmerman 2010) Change in community composition (Poff and Zimmerman 2010) Terrestrialisation or desertification (Poff and Zimmerman 2010) Reduced area of riparian cover (Poff and Zimmerman 2010) Reduced riparian recruitment (Poff and Zimmerman 2010) Invasion of exotic riparian species (Poff and Zimmerman 2010) Increased mortality/reduced growth rates (Poff and Zimmerman 2010) Reduced species richness Reduced cover (Poff and Zimmerman 2010) Increased variability Decreased germination/survival and growth (Poff and Zimmerman 2010) General soil moisture shortage Trees: Slower Growth/ Mortality Dieback (Jensen et al. 2008), (Roberts 2004), (Cunningham et al. 2009) Less reproduction (Roberts 2004) Understorey: depletion of seed banks (Roberts 2004) Attrition of perennating organs (Roberts 2004) Differentiation in dieback (Cunningham et al. 2009) Changes in Xylem water potential (Merritt et al. 2010) Wilting, chlorosis, leaf discoloration, leaf death, canopy reduction (Merritt et al. 2010) Terrestrialisation (Roberts 2004), (Overton et al. 2006), (Wen et al. 2009), (Kingsford and Thomas 2004) Loss of vigour/cover of dominant trees (Roberts 2004) Lower species richness (Roberts 2004) Reduced number of strata (Roberts 2004) Reduced leaf area (Capon et al. 2009) Increased species richness (Roberts and Hale 2007) Abundance and proportion perennial species (Roberts and Hale 2007) Increased exotic species richness/abundance (Roberts and Hale 2007) Fewer peak flows Decreased duration of floodplain inundation Loss of seasonal flow peaks General Water availability 24 Decreased water dispersed species (Roberts and Hale 2007) Decreased forest productivity (Horner et al. 2009) Vigour/Decreased Leaf Area (Horner et al. 2009), (Cunningham et al. 2007b), (Wen et al. 2009), (Roberts 2004) Dieback (Cunningham et al. 2009) Increased salinity from reduced leaching (flushing) Reduced riparian vegetation health Increased Black Box distribution (Holland et al. 2009), (Overton et al. 2006) Increased salinity from lower water tables Species loss (Salter et al. 2008) Riparian Vegetation condition (Overton et al. 2006) Terrestrialisation (Overton et al. 2006), (Ringrose et al. 2007) Changes in tree and shrub height (Ringrose et al. 2007) Crown condition (Wen et al. 2009) Fewer Floods Table 4.4.2: General measures of health used for riparian or floodplain vegetation. General Vegetation health Indicator How measured References (Lobo et al. 1997) Ecological Condition Hectares of dead trees (Roberts 2004) Nativeness % of species that are non-native (Roberts 2004) Canopy Condition % Canopy alive (Roberts 2004) Regeneration Density Number of juveniles/seedlings/ Hectare (Roberts 2004) Demography Number regenerating tress in size classes (Roberts 2004) Plant Area Index the area of leaves and stems per unit ground area, (Cunningham et al. 2007b) Photosynthetic capacity/Vigour/Biomass NDVI (Cunningham et al. 2007a; Cunningham et al. 2009; McVicar et al. 2003; Sims and Thoms 2002) 25 4.5 Biological Processes Functional integrity is a complement to structural integrity and refers to the rates, patterns, and relative importance of different ecosystem-level processes (Gessner and Chauvet 2002). The biological processes of metabolism, respiration and primary production respond to environmental variables that are commonly influenced by catchment disturbance (e.g. such as light and temperature regimes and nutrient loads) (Bunn et al. 1999; Fellows et al. 2006). Community attributes such as macroinvertebrate functional indicators can be used to track ecosystem processes (e.g., secondary production, leaf processing rates) in the case of extreme disturbance, but may not be sensitive at lower levels of disturbance (Bunn and Davies 2000). Therefore, direct measures of ecosystem processes, such as benthic community metabolism, are important considerations in aquatic ecosystem health monitoring (Bunn and Davies 2000; Fellows et al. 2006). Ecosystem processes that potentially could be used as functional indicators of river ecosystem health include: Rates of leaf breakdown Ecosystem metabolism (the combination of algal productivity and ecosystem respiration) Rates of nutrient uptake Microbial respiration Nitrification Fine particulate organic matter export Coarse particulate organic matter retention Invertebrate production (Young et al. 2008). Of the above, rates of leaf breakdown and ecosystem metabolism are more commonly used because they respond to many physical and chemical stressors and are relatively inexpensive and easy to measure (Young et al. 2008). There are no studies looking at the relationships specifically between water scarcity and process measures such as benthic metabolism or leaf litter decomposition. 26 Process responses like leaf breakdown and ecosystem metabolism are affected by a wide range of factors, both anthropogenic and natural (Gessner and Chauvet 2002; Young et al. 2008). In some circumstances (general monitoring) this can be a bonus, adding a range of detectable impairments to the detection. On the other hand if can be an impediment to specific impact monitoring because of the problem of partitioning out the effects of the impairment of interest (Young et al. 2008). For example, leaf litter breakdown has limited sensitivity and robustness because it responds to multiple factors that complicate the partitioning of effects from anthropogenic stress and natural variability (background noise) (Gessner and Chauvet 2002). Rivers at different altitudes or in different ecoregions also show systematic variation in naturally occurring nutrient concentrations, hydrological regimes, river geomorphology and bed substrate, and the nature of the riparian vegetation (which influences stream temperature via shading) hence water temperature and ultimately leaf decomposition rates (Young et al. 2008). Litter breakdown assays can overcome this limitation by being used in an impact monitoring situation (e.g. downstream–upstream comparisons) (Gessner and Chauvet 2002), or sensitivity can be generally improved statistically be measuring covarying effects such as water temperature, light, or nutrient concentrations in the field and partitioning them statistically (Young et al. 2008). Similarly, to account for as much external variability as possible, these methods demand highly standardised protocols for sampling and measurement (Gessner and Chauvet 2002). Either way, the need for removal of other factor effects by sampling or analysis reflects the suitability of the type of indicators for small scale rather than regional monitoring. Water scarcity may contribute to higher levels of, say, primary productivity but reference sites without the water stress would be needed to partition out the water scarcity effects (as per Figure 3.2a). Responses to hydrological changes described in Table 4.5.1 are observed, or predicted, from existing literature. It should be noted that Young et al. (2008) looked at many more influencing factors than just flow, and all of the listed responses can occur for a multitude of non-flow related reasons. 27 Table 4.5.1: Responses of biological processes to reduced flow or discharge, including drought. GPP = primary productivity, ER = ecosystem respiration, P/R = ratio of photosynthesis to respiration (P/R). All are from Young et al. (2008). Flow characteristics affected Biological or Ecological Response Reference Water Abstraction (reduced flows) Changes in Leaf Breakdown? Increased GPP, P/R Increased GPP Young et al. (2008) Young et al. (2008) Gessner and Chauvet (2002) Flow fluctuations (Floods) Decrease in GPP, P/R, minor decrease in ER Young et al. (2008) Increased River Drying Increased GPP, P/R Young et al. (2008) High flows and Abrasion Reduced Algal Biomass Young et al. (2008) Fellows et al. 2006 28 5 Potential suitable indicators for ecological indicators of water scarcity from responses cited in the literature In this section I merely list all of the potential indicators as found in the literature review and speculate on the data needed to convert the response into an indicator and issues that may influence its use. 5.1 Macroinvertebrates Table 5.1: Data requirements and issues identified for generating indicators for the ecological responses identified in the literature review to a potential indicator for water scarcity related stress for macroinvertebrates. Potential indicator Data needed Issues Decreased density (and biomass) Time series of density or historical density estimates What is a natural density? There are natural cycles in density associated with the nature of our streams anyway Decreased Richness Expected richness without stress Coarse indicator that changes with many stressors Decreased Diversity Expected diversity without stress Coarse indicator that changes with many stressors Changed composition (incl. Salinity/pollution tolerance, functional guild ratios, etc.) Expected composition without stress. Long-term reference data sets documenting natural variation. Need comparison sites that are subjected to the same stressors excepting hydrological stress. Need to understand which Australian taxa are affected (further research needed). Responses likely to be site or habitat specific Increased Drift Invertebrate drift estimates and a reference – historical data or reference sites Drift is only occasionally measured. Confounded when scarcity turns into no flow. 29 5.2 Fish Table 5.2: Data requirements and issues for getting from ecological response from literature review to a potential indicator for water scarcity related stress for fish. Potential indicator Data needed Population declines Spatial and temporal records to (abundance and account for natural variability Issues Also related to other stressors biomass) Fish kills Event based monitoring Can be caused by other agents (blackwater, erosion after bushfires) Decreased reproduction Recruitment/demographic data Spatially confounded, spawn in including natural/historical values different areas in different years? Changed communities Spatial and temporal records to Further research may be (incl. increased invasives, account for natural variability needed to isolate water and guild changes, IBI scarcity type responses scores) Altered movement Tagging/tracking data, need for Probably dependent on a patterns historical information to determine multitude of other factors, such natural movements as flow history, barriers, etc. Reduced species Spatial and temporal records to Also related to other stressors richness (incl. local account for natural variability extinctions) 30 5.3 Algae/Plankton Table 5.3: Data requirements and issues for getting from ecological response from literature review to a potential indicator for water scarcity related stress for Algae/Plankton. Data needed Potential indicator Issues Increased/altered Reference biomass values that Very noisy and skewed algal/phytoplankton account for cyclical nature of the distributions making sensitivity biomass response low. Lack of historical data. Altered benthic Reference assemblage data with Extremely difficult to convert community or historical natural changes community type measures to zooplankton assemblage indicators of known and meaningful response Reduced production As per biomass As per biomass Decreased diatom Knowledge of which species are Can respond to other stressors richness and diversity salinity tolerant/sensitive if present 5.4 Miscellaneous biota Table 5.4: Data requirements and issues for getting from ecological response from literature review to a potential indicator for water scarcity related stress for miscellaneous biota. Potential indicator Reduced migration Data needed Issues Need historical information to Probably dependent on a determine natural movements multitude of other factors, such as flow history, barriers, etc. Altered size class Historical/reference size class data distribution Reduced reproduction Difficult to convert to meaningful indicator Historical reproduction Possibly a long-term response, volumes/frequencies, etc not currently monitored anyways Decreased propagule Historical/reference data richness Decreased reproduction Natural phenomenon in ephemeral systems Historical reproduction Long-term, variable, tedious to 31 (turtles) volumes/frequencies, etc determine sensible indicator, not available everywhere Hormonal responses Long-term records of natural Time consuming and responses expensive to collect. Not known to be sensitive anyways Increased emigration 5.5 As per migration As per migration Waterbirds Table 5.5: Data requirements and issues for getting from ecological response from literature review to a potential indicator for water scarcity related stress for waterbirds. Response Data needed Issues Increased/Decreased Waterbird Abundance Accurate population estimates, Long-term data with seasonal fluctuations Migration, etc. Controls for other variables that affect abundance? Site specific and low abundance doesn’t necessarily mean poor condition Decreased Waterbird Diversity Expected diversity without stress Naturally temporally variable, difficult to determine what is a significant change Changed community composition Pre-stressor community composition, measure of natural variability in community Very difficult to turn into an indicator. Natural variability very high Increased migration Background migration levels without water stress Confounded by conditions elsewhere? Can’t migrate if not present anyway. Change in diet Expected diet. Frequent sampling. Quantifying diet? Or just a switch? Is it water stress causing switch? Increased/Decreased waterbird breeding Long-term breeding records (bird numbers, species, locations, etc) What is normal breeding? Is it the number of species breeding? Number of individuals breeding? Decreased Breeding success Historical breeding attempts, fledgling success, nest abandonment records, What is natural? Is water stress the only limiter? Is persistence already an indicator? Reduced Breeding events Long-term records of breeding events for specific sites Is it number of events through a period of years? 32 5.6 Vegetation Table 5.1: Data requirements and issues for getting from ecological response identified in literature review to a potential indicator for water scarcity related stress for macroinvertebrates. Response Data needed Issues Increased/Lower species richness Long-term records for each monitoring site/spatial unit Probably naturally highly variable between sites – Very difficult to set reference condition, let alone decide what is a significant change unless there is substantial historical data available Vegetation encroachment, Increased/Reduced Riparian cover Records of natural distribution for every site/spatial unit. Including documented natural changes without water scarcity Lots of external interventions also affecting this. Responds to other stressors. Requires loads of surveys and data. Change in community composition Pre-stressor community composition, measure of natural variability in community Very difficult to turn into an indicator. Natural variability very high Increased wood production/Decreased forest productivity Very long-term data sets Difficult to imagine how enough precision could be achieved to give reasonable sensitivity Terrestrialisation or desertification Long-term species distribution data for each site/spatial unit Already varies with distance to channel/water, so may be difficult to partition natural variation from water stress responses Reduced riparian recruitment Natural recruitment records including density and frequency Fine scale measurements needed. Difficult o to imagine sensitivity at large scales Increased mortality/reduced growth rates Comparison mortality/growth rates Tedious to measure growth rates, but otherwise promising. Can dead veg be easily monitored? Lidar? Decreased germination/survival Comparison germination/survival rates Tedious to measure rates, Longterm needed Depletion of seed banks Historical/natural data needed for reference. Tedious to measure, long turn around for results Attrition of perennating organs Non-water stress related attrition rates Could be confounded by other stressors anyways Dieback/Canopy Condition/ Plant Area Index/ Photosynthetic capacity/Vigour/Biomass Non-water stress related values, historical and spatial references Known to vary naturally temporally and spatially Differentiation in dieback Stand density and age data Already shown to not work for Changes in Xylem water potential Reference values. Long-term data Tedious for little reward at large scales. Clouded by within stand variation Loss of vigour/cover of dominant trees Long-term cover and species data. Species data still needs on ground monitoring at this stage Reduced number of strata Long-term data needed Hard to determine reference and a sensitive indicator. Probably very site specific Abundance and proportion Reference conditions required, Tedious to measure E. camaldulensis 33 perennial species probably from long-term data Increased exotic species richness/abundance Long-term data with species identifications and distributions Tedious to measure. Small scale Decreased water dispersed species Long-term data with species identifications and distributions Tedious to measure. Small scale 5.7 Biological processes As noted by Fellows et al. (2006), the most important feature of a good indicator of ecosystem health is that it responds to the disturbance gradient of interest. The nature of ecosystem process measures mean that they respond to multiple stressors and are therefore not considered in this section. 6 Current Status of Ecological Indicators Data sets listed in the “Raising National Water Standards project Ecological Outcomes of Flow Regimes” project (Overton et al. 2009) were investigated for each group. Furthermore, jurisdictional data sets provided by project 2a and in previous reviews by Gippel (2007) and Edgar (2008) were interrogated. Programs or data sets that do not use appropriate sampling strategies cannot be considered. For example, research has shown that sites chosen by expert opinion to be ‘representative’ are usually not representative. So, only data that are from a true probabilistic sampling strategy can be considered. Data from targeted monitoring programs, such as to monitor specific impacts, are also not suitable for this purpose. 6.1 In-stream biota 6.1.1 Macroinvertebrates Macroinvertebrates are widely monitored throughout Australia and generally follow sound sampling design and rigid defensible sampling protocols. There are programs in all states and these are well documented by project 2a. 34 It is rather unfortunate that so much of the potential value of macroinvertebrate data collected in Australia is untapped. Most programs rely on indicators that have very coarse responses to very coarse stressors. Even with the amount of investment in these programs, the indicators used have rarely been exposed to rigorous testing and validation of sensitivity. Indicators like SIGNAL2 and OE50 Scores are general responses that could not tease out water scarcity effects from confounding stressors, even if they were proven to be responsive. For example, the Ausrivas OE50 score was not related to hydrological indicators in a study looking at downstream of dam impacts (Marchant and Hehir 2002). Hence neither SIGNAL2 nor AUSRIVAS OE50 was identified in the literature as potential indicators, which included: Decreased density (and biomass) Decreased Richness/Diversity Changed composition (incl. salinity/pollution tolerance, functional guild ratios, etc.) Increased Drift. The first three of these indicators could readily be calculated from existing data sets because of the sound sampling strategies used. However, all of them would also respond to many other stressors than water scarcity and so have little value to this project. Increased drift is not monitored as part of any ongoing program at the moment. The use of specific tolerances and functional guilds is widespread overseas but sparsely used in Australia. If there is a way forward for bugs in this project it is via this route. 6.1.2 Fish Fish are monitored in the Sustainable Rivers Audit (MDBA), the whole of Victoria and NSW using the SRA sampling protocol, and will be sampled in QLD as part of the SEAP. There is a historical data set for 10 Queensland catchments using a suitable sampling strategy as part of the Long-Term Monitoring Program (LTMP), which is paused since 2007. None of the other states have existing programs that could produce suitable data for unbiased 35 environmental assessment. There were 81 fish data sets from the MDB recognised in the Ecological Outcomes of Environmental Flows Project (Overton et al. 2009). Of these, none are better than the above although some, such as the fishways assessment program, could be worth further investigation if needed. Of the potential indicators from (Table 5.2), the following indicators could not be calculated using existing agency data: Fish kills Decreased reproduction (this may be included in a revised SRA fish protocol within a couple of years) Altered movement patterns. The following could be calculated for fish using the existing programs: Population declines (abundance and biomass) Changed communities (incl. increased invasives, and guild changes, IBI scores) Reduced species richness (incl. local extinctions). All of these potential indicators would be expected to be very susceptible to many other stressors as well as water scarcity. 6.1.3 Algae and plankton Some state water quality programs include variables like Chlorophyll-a and/or algae counts and these are at various scales of spatial replication. There are 6 sites measured annually for algae counts in the Murray river by the EPA in South Australia, and up to 120 sites measured for algal counts twice yearly in the south East Queensland EHMP (Gippel 2007). It is presumed that there are also many regional monitoring programs carried out by councils, particularly where the resource has a high public use component. Only the 36 EHMP program collects data in a way that allows for meaningful scientific analysis on a monitoring program. 6.1.4 Miscellaneous As the name of this component suggests, there are only small scale and adhoc monitoring using the miscellaneous taxa. 6.2 Waterbirds Waterbirds are not included in any of the existing state or federal monitoring programs (Edgar 2008; Gippel 2007), nor are they listed in the Framework for Comparative Assessment of the Ecological Condition of Australian Rivers and Wetlands (Norris et al. 2007a). They are listed as of potential future value “when appropriate data become available” in the Assessment of River and Wetland Health: Potential Comparative Indices (Norris et al. 2007b). There are 89 bird related data sets listed in the Ecological Outcomes of Flow Regimes metadata spreadsheet. The majority are of limited spatial extent and are usually specific to a particular wetland or floodplain. Many have temporal replication, however, and therefore offer a good resource for further investigation. The only Australia-wide data sets are held by Birds Australia and are of limited value for monitoring because of inconsistencies in sampling protocols. Surveys of waterbirds with a recent temporal component generally do not include breeding observations. There are many data sets listed that record breeding observations, yet the majority of these do so only in an opportunistic way, i.e. only recording when breeding was noticed, and sampling effort is therefore inconsistent and incomparable. Surveys that record when birds do breed are recording when a system is not in stress, not when a system is in stress. This method can only be relevant when all systems are surveyed for breeding and using a consistent sampling method. There were five datasets that contain long-term information with a scientifically robust sampling design that remain contemporarily relevant (Table 6.2.1). Two 37 of these data sets are in the Coorong, two are across NSW or the MDB and one is the Narran Lakes system within the MDB (Table 6.2.1). Table 6.2.1: Contemporarily relevant waterbird data sets identified from the ecological response to flows project metadatabase (Overton et al. 2009) that could have value to the ecological indicators of water scarcity project. Data set Frequency Area covered Dates Notes Coorong waterbirds Annual (January) Coorong 2000 - present Adult counts, Bird food resources Eastern Australia Waterbird Survey Annual (October) NSW 1983-present Adult counts Eastern Australia Aerial Waterbird Survey Annual (October /November) MDB = NSW, QLD, VIC, SA, ACT 1983-present Adult counts, (species or functional group), Breeding Wader Surveys ?Annual Coorong and SE coastal lakes, SA 2000-2008 Shorebird counts Waterbird surveys of Narran Lakes Annual Narran Lakes, NSW, Lower Balonne, QLD 1970’s present Counts, Breeding, Habitat modelling The Narran Lakes data set is freely available, the Wader Surveys in Coastal SA belong to a jurisdiction (DEH) and the others are held by Academics. MDBA Icon Site Monitoring Program The MDB Icon site monitoring program has benchmarks for particular sites, such as minimum of three breeding events in 10 years (MDBC 2008). Further enquiry suggests that these ‘rules’ were arbitrarily probably put forward by local action groups prior to the ministerial approval of the TLM project in 2002 and are subject to review (Swiripek 2010). 38 6.3 Vegetation For most of Australia there are no ongoing systematic monitoring programs for riparian or floodplain vegetation condition. There will be vegetation surveys as part of the SRA and ISC (Victorian Index of Stream Condition) in the near future. There have been baseline surveys of vegetation in the Murray Darling Basin Authority TLM Icon sites and most of the Murray channel in South Australia. The Living Murray program has identified remote sensing as a valuable tool for monitoring and has implemented a “Mapping Stand Condition” project in 2008. The first report from this GIS based project should be available within a couple of months (J Swirepik, pers. Comm.). Of all the possible indicators given for vegetation responses to water scarcity, most require small scale on ground measurements, meaning extremely tedious numbers of measurements. This can’t be avoided by taking only a few samples at a lot of sites because there is so much within stand variability. However, it is so simple to take whole of stand assessments of health under the umbrella indicator of Dieback/Canopy Condition/ Plant Area Index/ Photosynthetic capacity/Vigour/Biomass using remote sensing. Remotely sensed data for parts of Australia has been acquired continuously since 1972 and since July 1981 for all of Australia daily (McVicar et al. 2003) and a brief description of the available data is given in Table 6.4. Access to some of these data sets would give very good spatial and temporal coverage, allowing for quite good determinations of regions suffering water scarcity. 39 Table 6.3: Technical specifications for major historical, current and future key terrestrial satellite systems as of September 18, 2003 (source: McVicar et al. 2003). The NOAA satellite data are used by the Bureau of Meteorology to report the NDVI for all of Australia every month using NOAA remote sensed data and 1.1 km pixels (http://www.bom.gov.au/jsp/awap/ndvi/index.jsp). This includes reporting NDVI anomalies, which indicates whether the vegetation greenness at a particular location is typical for a certain time of year or whether the vegetation is more or less green (http://www.bom.gov.au/climate/austmaps/about-ndvi-maps.shtml). At the moment anyone can access images, but the proposal is that end users should be able to access the layers themselves (King 2003). The MODIS data (LPDAAC 2010) are now freely available for the whole of Australia in grabs every 16 days since 2000 at 250m resolution. The MODIS data can be accessed by anyone and requires no pre-processing as EVI and NDVI are already calculated (Paget and King 2008). MODIS is the recommended way forward for this project and is further discussed in the next section. 40 6.4 Biological Processes Whilst a more complete assessment of river health would include functional indicators, (Young et al. 2008), direct measurements of ecosystem processes are often neglected in river health assessment programs (Bunn and Davies 2000; Fellows et al. 2006). The only functional metric that is used routinely in water-quality assessment is biochemical oxygen (O2) demand (BOD) and is most suited to sites influenced by wastewater discharges (Young et al. 2008). 7 Identification of indicators feasible to use, based on criteria including whether adequate data exist to populate the indicator. 7.1 Introduction Most of the available data sets have limited spatial extent and therefore different ecological indicators may be used in different parts of Australia. This is sensible as different ecosystems have different key components and the nature of what is being measured already probably reflects what is important in a region anyway. The issue of scaling the indicator to the scale of reporting is addressed using examples in section 8.4. 7.2 In-stream Biota 7.2.1 Macroinvertebrates There are no indicators feasible for macroinvertebrates to indicate water scarcity (alone) at this stage. All available indicators, or indicators that could be calculated form existing programs, are general health indicators and therefore susceptible to confounding. There is some potential for development of specific water scarcity indicators using existing data but this will require considerable future research (see Chapter 8). 41 7.2.2 Fish There are no indicators feasible for fish to indicate water scarcity (alone) at this stage. All available indicators or indicators that could be calculated from existing programs are general health indicators and therefore susceptible to confounding. 7.2.3 Algae and plankton There are no indicators feasible for algae and plankton to indicate water scarcity (alone) at this stage. Very few data are available and only the EHMP data set offers enough rigour in its design to allow further research into potential indicator development if desired. This region however has a subtropical climate and the findings may therefore not be totally relevant to the rest of Australia. 7.2.4 Miscellaneous There are no indicators feasible for the miscellaneous taxa to indicate water scarcity (alone) at this stage. All indicators that could be developed would require considerable investment into further data collection prior to development. 7.3 Waterbirds A proposal by Kingsford and Lee (2007) to the then Murray Darling Basin Commission suggested birds could be included as indicators of floodplain the health. The report suggested species richness, abundance and breeding as potential indicators. There was no documentation of indicator calculations, indicator variability, and indicator responsiveness or indicator demonstration. Hence the use of waterbirds as indicators of ecosystem health has a very long way to go, let alone the narrowing down of the responses to water scarcity when there are multiple stressors present. This taxa faces additional complex hurdles associated with spatial and temporal variability. On the other hand, these taxa may be well suited to event based monitoring. When an inundation event that triggers breeding is recorded, the success of breeding may be an indication of stress. That is, the number of breeding pairs, fledgling survival, nest stranding, nest desertion, etc, are all potential 42 indicators. However it is a long way from a potential indicator to an actual indicator. For every proposed indicator a series of questions need to be answered. What proportion of fledgling success is natural? What proportion is indicating water related stress? What is the variability of this indicator? How large does a difference in proportions need to be before I can statistically detect the effect? What is the responsiveness of this indicator? How can we separate water related stress from other stressors like chemical pollution and predation? Does setting up this indicator require an additional cost to existing programs? Are there sufficient data to set up a demonstration of this indicator? There are no feasible indicators of water related stress using waterbird data from existing programs. 7.4 Vegetation On-ground data collection for vegetation is not an option because of the tediousness of it and the problem of upscaling it to a meaningful indicator. The Victorian ISC has revised their streamside zone component and may be implementing statewide monitoring of the riparian vegetation using remote sensing. This will be worth following up on. At the moment, remote sensing and NDVI in particular appeals, and should be followed up. 7.5 Biological Processes There are major problems from indicators being confounded because of responses to multiple stressors that rules processes out at this stage. 43 8 Options and recommendations for the way forward 8.1 Introduction In this section I briefly list what would be required to go forward with the indicators, with the most potential from each group. I take the one indicator with the most potential, NDVI, through a detailed example of how the next step may progress. 8.2 In-stream Biota 8.2.1 Macroinvertebrates At the moment the analyses for river health type programs uses community level indicators like OE Score and SIGNAL score that have coarse responses to multiple stressors. The only way forward for macroinvertebrates as indicators of water scarcity related stress in Australia using currently available data sets requires further research investment. This would require extensive review of current data sets in conjunction with hydrological data, then further experimentation to determine which individual taxa or functional groups are suitable indicators. This would be a high-risk investment because it is probable that these groups, if they exist at all, are already affected or have limited distributions, and even then they may also be open to confounding from other stressors. A start would be to interrogate some existing macroinvertebrate data that has associated hydrological data. The SRA data at the valley process zone level appeals intuitively but would be expected to suffer from the coarseness of the macroinvertebrate measures used. This exploratory analysis was performed for the 2007 SRA data using a Spearman’s rank correlation coefficient of the 3 macroinvertebrate submetrics, and overall River Health Macroinvertebrate (RHM) metric against the five hydrology sub-metrics and the total hydrology (SRHI) metric (Table 8.1). The signal sub-metric was somewhat more related to the hydrology than the other metrics, but none were statistically significant at the 0.05 level. 44 Table 8.1: Spearman’s correlation coefficients for SRA macroinvertebrate metrics and hydrological metrics for 23 zones sampled in implementation period 3 (2007). OE = Ausrivas OE50, S = Species Richness, RHM = SRA Macroinvertebrate health score, SRHIvar = Hydrological variability submetric, hf = High Flows, ls= low spells, lz= duration of zero flows, vol= Volume. SRHIvar SRHIhf SRHIs SRHIlz SRHIvol SRHI OE 0.19 0.09 0.12 0.20 0.01 0.11 S 0.12 0.17 -0.09 -0.13 0.20 -0.01 SignaL2 0.38 0.41 0.30 0.34 0.34 0.38 RHM 0.26 0.30 0.07 0.05 0.26 0.16 In other words, to determine likely indicators will require extensive hydrological knowledge, and if that is available then why use the bugs? The macroinvertebrate indicators (this applies to all ecological indicators) would only be of value in regions where hydrological data wasn’t available. A simpler alternative option is discussed in Chapter 9. The response of individual taxa or functional groups may offer better value as indicators and if this theme were to progress, the next step would be to interrogate the relationships at that level. This would require considerable effort (and expense) for a potentially weak response and is therefore described as a low priority. A related type of analyses at that finer taxonomic scale has recently been looked at in NSW for the long-term relationships with temperature (climate change) by Bruce Chessman (Chessman 2009). A major difference is that water scarcity is probably not a long-term gradual effect as climate change was treated for that paper. Recommended way forward:- Drop macroinvertebrates as a potential indicator of water related stress in Australian ecosystems. 8.2.2 Fish The best way forward for fish would be to perform research to attempt to isolate indicators that are water scarcity specific. This is intuitively a waste of resources. Fish function in ecosystems is very complex and their response to 45 water scarcity in amongst so many other influencing environmental factors could be best described as unpredictable. Like waterbirds, absence doesn’t mean a lot and besides there are no sampling programs that detect every fish every time, so probability of absence needs further work. Fish monitoring is not performed in a representative way outside of the MDB (plus the remainder of Victoria and NSW), therefore it hardly seems worth investing more resources in this group for this project. I interrogated the SRA data at the Valley process level. This involved testing for relationships between the hydrology (FSR) data and the individual fish theme sub-metrics. The SRA themes are set up to report on different aspects of the ecosystem, so correlations between the fish and hydrological scores for each catchment are unlikely, however for fish there are 13 sub-metrics calculated for each zone, which are then combined into nativeness, diagnostics and expectedness, which are then combined into a fish health metric. This analysis was not expected to show significant relationships, as a study had already shown the lack of response of fish indicators to hydrological variables in one part of the MDB (Growns 2008). Of the five FSR sub-metrics used in the SRA, the one most likely to surrogate for water scarcity is SRHIvol, which reflects the total volume of water available in the system during the past 5 years, and compares this to a natural value. A high score reflects the region has little modification from natural and a low score means the total volume is significantly less than natural. The highest relationship of any of the SRA fish submetrics to SRHIvol was a rank correlation of -0.34 (Table 8.2). This suggests very weak relationships and that factors other than hydrology play a major role in the SRA fish submetrics. Graph of the relationships confirm that the fish scores are quite independent of the total volume indicator. The example graph I include is the one with the strongest relationship, the Observed to Expected (OE) proportion of historically occurring native fish in the region (Figure 8.2). It clearly demonstrates a weak relationship exists but high or low OE scores can be associated with high or low hydrology scores (Figure 8.2). 46 Table 8.2: Spearman’s correlation coefficients for SRA fish theme submetrics and hydrological total volume metric for 23 zones sampled in implementation period 3 (2007). Negative values mean that when total volume is closer to natural the fish indicator performs poorer. Indicator Correlation with FSR Volume indicator -0.19 -0.34 -0.03 -0.07 0.34 proportion native abundance -0.24 proportion native species proportion native biomass proportion macrocarnivores proportion mega carnivores total abundance fish with abnormalities -0.25 -0.06 0.13 -0.15 -0.11 0.22 zone weighted caught to predicted ratio native species 0.02 Fish total species richness observed to expected ratio pelagic species richness benthic species richness intolerant species richness Figure 8.2: Relationship between SRA hydrology total volume metric and SRA Fish OE score at the valley zone for 2007 (IP3). Arrow and shades merely represent arbitrary cut off for healthy OE scores. 47 Recommended way forward:- Drop fish as a potential indicator of water related stress in Australian ecosystems. 8.2.3 Algae and plankton There is no obvious way forward. 8.2.4 Miscellaneous There is no obvious way forward. 8.3 Waterbirds Existing indicators do not exist but there are some data sets available, as well as the potential to develop event-based indicators. Waterbird data, especially counts, are highly variable spatially and temporally and therefore are extremely unlikely to be sensitive to detecting anything but very coarse changes. Waterbird indicators also have limited relevance in many parts of Australia and in many years. Because there is a high likelihood of getting indicators with very low sensitivity, any further research into using waterbirds for this project are classified as quite risky. An inexpensive way forward may be to fund some small projects looking at developing event-based indicators using existing data sets. This is seen as a low priority. A more expensive way forward would be to fund some projects looking at developing annual indicators using existing data sets, with a view to eventually become a partner supporting ongoing annual waterbird surveys. This is seen as a very low priority. Recommended way forward:- Drop waterbirds as a potential indicator of water related stress in Australian ecosystems. Use existing waterbird data to set hydrological threshold/trigger values rather and monitor hydrology and hydraulics. See alternative approach (Chapter 9). 8.4 Vegetation The general health of the vegetation, as measured by NDVI using remote sensing, appeals as a sensible way forward. Other factors contribute to changes in NDVI, such as herbivory and seasonal variations, but the use of 48 reference sites and occasional ground-truthing would be simple. The data have an archived and accessible history and therefore could be used in conjunction with historical soil moisture/hydrological records for calibration. MODIS (LPDAAC 2010) derived data is freely available (Paget and King 2008) and a suggested way forward would be to interrogate these data to look for responsiveness and sensitivity of NDVI to water related stress. An issue is the sampling unit and reporting unit. Currently all of Australia is sampled, but it seems sensible to only sample and report on riparian and floodplain vegetation. This would require using reporting GIS layers to excludemajor chunks of Australia from the reporting. It would also allow reporting at any scale desired, such as specific wetlands or rivers or SWMA, as defined for the FARWH. A sensible approach may be to relate archived images against archived hydrological data including floods etc. Then calibrate and test the functioning of the images against known hydrological stresses and then apply the findings across all SWMAs or chosen reporting units for the chosen reporting period. It is important to have spatial reference regions for comparisons. That is, some regions that are known not to be stressed by water scarcity should be used to monitor natural fluctuations in NDVI. 8.5 The way forward using NDVI This section is written in anticipation of the NDVI being trialled as a way forward, but could apply in general to any chosen indicator. Source of the data Whether it’s a biological indicator or NDVI, there are several data sources available and these should be compared to determine the best individual source, remembering that multiple sources may be possible. For example, some suggested criteria for sourcing data for the NDVI indicator are given in 49 Table 8.5.1. For NDVI, MODIS is clearly the preferred supplier of data. All the information required to use the MODIS NDVI data for Australia is contained in the paper by Paget and King (2008) and is included at the end of Appendix 2. Table 8.5.1: Example of criteria to determine best possible sources of data for population of ecological indicator of water scarcity related stress. *Each source is rated Low Medium or High for these criteria. ? means further research such as a pilot study is required. NOAA Landsat MODIS Yes Yes Yes Yes No Yes At what spatial scale is the measurement made? 1km ?100m 250m At what temporal scale is the measurement made? daily ? 16 days ? ? ? M L H H H H ? ? H Availability Are the data available for the temporal and spatial components required (say 2000 – 2008) Future relevance Are the data going to be available/relevant/comparable with future sampling/monitoring? Resolution Sensitivity Are these data able to demonstrate a change in response to water scarcity at the reporting scales required? Ease of use* Is it relatively simple for the data to be analysed and interpreted? Accessability* Is it relatively easy to access the data? Cost What is the cost of obtaining the data? (H=low cost) 50 Spatial considerations Spatial scale is probably the most important consideration needed for the entire project. There are two aspects: scale of measurement and scale of reporting. NDVI again appeals here because it is measured at 250m pixels and can be scaled up using averages to any scale, even the entire country or entire MDB (Figure 8.3). Figure 8.3: Example NDVI time series for the entire MDB (Schmidt et al. 2005). Remote sensing of vegetation has specific peculiarities of scale to consider. These include whether to extract just flood plain data, or just the riparian zone data? The whole of catchment data (as in Figure 8.3) will return averages that are heavily influenced by agriculture and whilst these may add some value I think just riparian/floodplain data would better reflect water scarcity ecological impacts. I believe there are GIS layers of riparian zones available, possibly just for the MDB and or Victoria as used in the SRA and ISC programs respectively. Access to these layers would substantially reduce the cost of any pilot study. 51 The chosen spatial unit for reporting should probably reflect the scale at which impairment is expected to be detectable. The reporting scale can be flexible and may reflect set areas, say 10 km2 grids, catchments, ecological regions, Surface Water Management Areas (SWMA), or sites of particular interest (e.g. a RAMSAR wetland). The users of the end product will decide on the scales of reporting to be used. The response of NDVI should be looked at using average values but could also include comparisons of shapes of the distribution within each spatial reporting unit. Temporal considerations The dates of the assessment period need to be determined. The data will probably need to be seasonally adjusted (NDVI definitely does) to make comparisons meaningful. This has consequences for the comparison to be made. For example, should one be comparing long-term trend or trajectory or just be looking for a step or drop in the NDVI anomaly. It would probably be sensible to look for a gradual decrease in the NDVI anomaly in the assessment area relative to the reference area(s). Another thought, however, is how to treat the anomaly temporally. It could be calculated on the 16 day cycles, monthly, or quarterly. A pilot study looking at a known stressed region should look at the effects of using different periods to calculate moving average of the NDVI anomaly, and see at what point the stress could be picked up. Reference considerations Are there data available for suitable non-stressed regions to act as references? The references could be based on similar hydrological zones, or similar ecological zones. It is also possible to simply analyse all regions in, say, NSW at the same time and merely rank or order them in terms of apparent NDVI anomalies for the reporting period. 52 A suggested method This is only a suggestion; there are many ways that an indicator may be used. Choose spatial and temporal reporting scales. e.g. The Lower Bidgee floodplain between 2005 and 2009 Choose one or more appropriate reference locations for the same period that are known not to be water stressed. Appropriate may mean subject to similar non-water scarcity stressors, such as latitude or land use, or it may refer to starting NDVI. Having similar starting NDVI is not essential but may simplify interpretation. Say, a coastal catchment that has not been drought declared during the sampling period. Calculate say monthly NDVI values for each region for the sampling period Seasonally adjust each series of data (using long-term data, not just the sampling period)1 Calculate NDVI anomalies for each series during the sampling period Perform an intervention analysis type approach to compare the NDVI anomaly series. Before using NDVI It’s all well and good to recommend using NDVI, however before any indicator is used, its responsiveness and sensitivity must be known. Therefore if it were This is easily done for NDVI data, e.g: Verbesselt J., Hyndman R., Newnham G. & Culvenor D. (2010) Detecting trend and seasonal changes in satellite image time series Remote Sensing of Environment, 114: 106-15. 1 53 desirable to look further into using NDVI as a potential indicator some validation trials should be run. These trials would involve collating information about regions that have been known to and not to be stressed during the 10 years that MODIS data are available for. The ability of the NDVI anomaly (or the EVI anomaly) to detect changes in those regions could be trialled in and the results used to inform future data collection and analysis. The US EPA Research strategy for the Environmental Monitoring and Assessment Program (McDonald et al. 2002) should be consulted throughout the trial process. 9 Alternative view At the end of the day the same question arises for any indicator or theme. Why use the ecological indicator if the hydrological one(s) are available? In other words, the only value that an ecological indicator could add is in a situation where there aren’t appropriate hydrological models, or if it is desired to determine measures of impact of stress rather than the level of stress. For example, it may be that a 10% alteration in flow severely degrades one ecosystem but a 30% alteration barely affects another. However, all the ecological indicators readily available respond to many other stressors as well, hence general ecological condition and hydrological stress are not always directly related. If it is the alteration to flow that is of primary concern then hydrological models will suffice; if it is impact then the ecological indicators may be adequate. On top of all this, it must be remembered that without further calibration, other stressors may affect ecological indicators. This project sets out to find ecological indicators by reviewing the literature and determining trigger points or values for an ecological response to occur. The condition of the taxon below these trigger values is used to set a 54 reference value against which to compare potentially stressed aquatic ecosystems. This requires monitoring the ecosystem in depth (pun intended!). An alternative approach could be to monitor the trigger values. In other words if the literature review has revealed that water bird species A in X region need a flood event of Y Megalitres at least once in every Z years to reproduce and persist in the area: Why monitor the birds? It is a huge cost and the indicators are not yet shown to be sensitive and their responsiveness is not known. We can model flood events practically anywhere in the country these days for comparatively little cost. If water stress alone is our concern then we could very easily monitor the hydrology/flood events to indicate whether region X has had enough water for a healthy ecosystem. If the birds don’t breed then that is probably of interest to someone somewhere, as it could indicate another stressor in the system, but water scarcity is not the issue. 10 Conclusion Of the tens of potential indicators identified in this paper, all are susceptible to confounding. In other words, factors other than water scarcity can influence the ecological response. Water scarcity can be measured directly using hydrological data and therefore should be used where available. If there are any regions where hydrological data are not available then NDVI as calculated from NOAA or MODIS satellite data appeal as an appropriate ecological indicator. Further research needs to be done to evaluate its response in different regions and at different scales but the data are archived and freely available to do this. Even NDVI, however, cannot be used in every corner of Australia and alternative measures for each region may need to be identified. The main recommendation from this paper is to not use ecological indicators to identify water scarcity related stressed ecosystems. Ecological indicators may be used to assess which stressed systems are in worse condition than others if required. 55 Appendix 1 Water birds literature review A1.1 Literature Review of Waterbird Ecology relevant to water use and water scarcity Waterbirds depend on wetlands Waterbirds depend on wetlands for survival, reproduction and recruitment (Bellio et al. 2009). When wetlands disappear for whatever reasons, waterbird numbers decline. For example, waterbird numbers estimated during annual aerial surveys collapsed by 90% when the Lowbidgee wetland was destroyed by development of the floodplain for an irrigation area (Kingsford and Thomas 2004). On the other hand the response is not always so obvious. Hydrological data indicate that river management from the Hume weir began impacting on waterbird breeding areas in the mid-1950s, but ornithological records reveal that the natural system resisted change for 20 years but changed after about 1975 (Lesley 2001). River and floodplain management River regulation and the disruption of the seasonal flood regime along a river often spells disaster for waterbirds (Nilsson and Dynesius 1994). A reduction in water flow in to wetlands results in reduced breeding events for colonial nesting bird species (Hughes 2003; Kingsford and Johnson 1998) and reduced breeding success for wading birds in general (Russell et al. 2002). Water alone is not enough to guarantee success as waterbird breeding can be directly linked to other factors like variability in flow (Briggs et al. 1997; Lesley 2001). Perhaps the most critical factor likely to affect the long-term stability of waterbird persistence is the interval between breeding episodes. This factor is of real concern during extended drought periods and managed forest flood events (Lesley 2001). For example, in the Barmah-Milewa forest, breeding numbers of egrets have not exceeded 330 pairs since the mid-1970s yet these species were recorded nesting in ‘thousands’ or in the ‘largest known egret rookery in Victoria’ in 1940 and 1961 respectively (Lesley 2001). 56 Whilst birds may occur on almost any water body, water following the natural flood regime will not support colonial water birds in the long term without suitable nesting habitat (Kingsford and Johnson 1998) and safe roosting sites (Paillisson et al. 2002). For example, altricial waterbirds (the young are cared for by the adults) nest on branches over open water in trees at the edge of River Red Gum areas or in dead River Red Gums (Briggs et al. 1997). The associations of breeding Darters, Great Cormorants and Pacific Herons with wetlands containing large areas of dead River Red Gum reflected this preference (Briggs et al. 1997). Wetlands provide a food source for waterbirds Wetlands provide an important source of food whether the birds eat plant material, fish or macroinvertebrates and all these food sources can be linked to flow. Some waterbird species are herbivorous and many of their distributions are related to wetland plant communities (Desgranges et al. 2006). Other species specialise in fish (piscivores) and their distributions are less related to fish community types but to abundance of appropriate sized fish. For some other waterbird species (generally waders), presence can be determined by aquatic macroinvertebrates abundance (Briggs et al. 1997). Within wetlands, water depth and fluctuations influence macrophyte and macroinvertebrate distributions - which ultimately determines waterbird communities (Bellio et al. 2009). Litter inputs, organic matter decay, the timing and level of nutrient releases and increases in invertebrate populations are linked to periods of submergence and desiccation associated with flow peaks and troughs (Lesley 2001). Fish population numbers follow the increases in plant and macroinvertebrate populations (Crome 1988) and fisheating bird numbers follow the fish population numbers (Kingsford and Auld 2005). Water birds use wetlands for breeding For successful breeding to occur, suitable nest sites, cover, and food must be available over a specific period of time (Kushlan 1993). Different bird species have different nest types (ground, floating, attached, or above water) (Desgranges et al. 2006) but reduced flows to wetlands adversely affects breeding habitat and subsequent breeding of waterbirds (Briggs et al. 1997). 57 Different species respond differently to different flood types, for example breeding waders need small scale floods (Eglington et al. 2008). Differences between species in breeding and recruitment may offer additional possibilities for measuring floodplain and river health for a catchment (Kingsford and Lee 2007). When do Water birds use wetlands for breeding There are many different potential cues for waterbird breeding to occur but generally when food resources reach sufficient abundance and waterbirds gain enough weight they will breed (Kingsford et al. 1999). This general rule applies across different regions and other cues. For example, breeding of Australian waterbirds coincides with food abundance in the southern spring, the wet season in the tropics and following floods inland (Kingsford and Norman 2002). Most breeding in south-Western Australia occurs in spring and rainfall was the most important proximate cue stimulating gonadal recrudescence (Halse and Jaensch 1989). Factors affecting the success of the breeding event In permanent or semi permanent wetlands, the reproductive success of waterbirds may depend on water level fluctuations to make food available during nesting (Kushlan 1993). In more ephemeral wetlands a flood event that triggers a breeding event does not guarantee the breeding will be successful. The response of the birds to the flood event are not instantaneous and low numbers of waterbirds usually occur after flooding, when habitat is extensive and waterbird numbers have not increased by immigration or breeding (Kingsford and Norman 2002). Waterbird abundance is usually at its highest during the drying phases of wetlands (Kingsford et al. 1999), for example numbers were greatest on Lake Eyre in December 1990 after the flood had reached the lake in August (Kingsford et al. 1999).The duration (and by association the rate of fall) of the water determines the reproductive performance of the colony (Lesley 2001). Changes in wetland area affects waterbirds In general the total number of birds in a wetland system is related to the total area of the wetland available as in general a larger wetland area means a 58 larger potential habitat range. Broadly speaking, water availability is related to wetland area and decreased wetland area equals decreased wader diversity (Green et al. 2002). Waterbirds are often distributed around the edge of wetlands where water is relatively shallow (Kingsford et al. 1999) and larger areas of shallow water therefore means larger total population size. Yet species respond differently to the hydrology of the system. For example, fluctuating water levels suit small to medium shorebirds that forage in < 30 cm water (Bellio et al. 2009) but any changes in water level can have a negative impact on breeding success of many wetland bird species (Desgranges et al. 2006). Reduced flow equals reduced wetland areas We know that flow is positively related to wetland area. (Kingsford and Auld 2005). The loss of total wetland area and subsequent habitat loss through draining of wetlands, regulation of rivers, diversion of water for irrigation and floodplain development are major threats to waterbirds (Kingsford and Norman 2002). This applies even when the wetland area may be the same or similar, but the hydrology of the forest has changed considerably. For example, we know that the Barmah-Millewa forest supported large numbers of colonially-nesting waterbirds prior to the commissioning of the Hume Reservoir (Lesley 2001). River management in the Barmah Milewa forest has reduced the frequency of the natural flooding regime which has resulted in 80% fewer successful breeding episodes (Lesley 2001). Reduced water however can result in large concentrations of waterbirds either in situ or when birds are forced to move and concentrate on the remaining, more permanent wetlands (Kingsford and Norman 2002). Other effects from hydrological modifications at the large scale is the changes includes the relative representation of wetland types and wetland habitats (Robledano et al. 2009). Changes in water management often means reduced flooding and flooddependent vegetation will die through lack of water and increased impacts from salinisation (Kingsford and Thomas 2004). These types of changes could conceivably favour piscivorous birds over herbivorous birds. 59 Flows and floods affect colony size For Australian waterbirds, food abundance usually coincides with flooding patterns or rainfall, which produce wetland habitats (Chambers 2008; Kingsford and Norman 2002). Again, the size of the flood event has different repercussions for different species. Before they respond by breeding, colonial waterbirds usually require the flood to reach a certain threshold (Kingsford and Auld 2005). This hydrological threshold can be related to flood high, timing of flood peaks, and duration of flooding (Lesley 2001). Naturally, successful breeding events contribute to overall colony size, hence it is documented that total colony size (number of nests) and sizes of six nesting Ciconiidae (Intermediate Egret, Rufous Night Heron, Glossy Ibis, Straw-necked Ibis, Australian White Ibis and Royal Spoonbill) colonies were significantly related to annual flows in the Macquarie Marshes in 1978, and 1986 – 1996 (Kingsford and Johnson 1998). Whilst the total volume of flow in a year was a good indicator of the extent of waterbird breeding in the Macquarie Marshes (Kingsford and Lee 2007) for most colonial waterbirds the relationship was also very strong for flow in just the three months before breeding (Kingsford and Auld 2005). Hence the timing of the flow is important and pulses rather than annual volumes also play a role. For example, in order to induce breeding in the Barmah-Millewa Forest, the pulse maximum had to be a minimum size and needed to occur in September and/or October (rarely November) (Lesley 2001). Habitat quality is related to hydrology The presence of safe roosting or breeding sites are mainly governed by the hydrological and disturbance conditions in wetlands (Paillisson et al. 2002). Reduction in water flow to wetlands reduces both semipermanent and ephemeral wetland vegetation (Hughes 2003). Changes in hydrological conditions lead to habitat changes in wetlands that can be related to changes in the ranges of colonial waterbirds (Kushlan 1993) and even modify the 60 surrounding bird community towards a more heterogeneous assemblage including scrubland and palustrine species (Robledano et al. 2009). With a reduction in flooded areas, there will be less feeding habitat for waterbirds and fewer breeding opportunities (Kingsford et al. 1999) and at the edge of open water, fewer trees that provide their nest sites (Briggs & Thornton 1995). Foraging success is the key to breeding Even on some semi-permanent Australian wetlands that contain cormorants most of the time, there is great variability in abundance and this presumably reflects food availability (Kingsford and Norman 2002) and colonies are less likely to breed successfully without sufficient resources. Prey availability after breeding has commenced may be the critical factor and it is known to be closely related to the growth of colonial waterbird chicks (Kushlan 1993). So, whilst waterbirds obviously need water for persistence, and habitat for nesting, foraging success may be the key to breeding success (Paillisson et al. 2002; Russell et al. 2002). The argument for wading waterbirds may be summarized as follows: Breeding activities are energetically demanding. Wading birds mainly consume aquatic vertebrates The efficiency with which these can be acquired depends in large part on the water conditions. Poor foraging efficiency leads to late initiation of nesting, high mortality of offspring, abandonment of nests, or any combination of the above (Russell et al. 2002). The principle applies to herbivores as well, as about 200 Black Swans died when aquatic macrophytes declined at Lake Altibouka (Kingsford and Norman 2002) whilst in the Netherlands the abundance and duration of stay of water birds are closely associated with the presence of Chara. (Noordhuis et al. 2002). Nesting of Australian Pelicans on Lake Wyara in south-western Queensland coincided with high levels of fish populations after a flood event 61 while Black Swans nested later as the water cleared and aquatic macrophytes became abundant (Kingsford and Norman 2002). Foraging success for survival (not breeding) Waterbird communities are ultimately determined by the distribution of the prey of the birds (Bellio et al. 2009). Similarly, the composition and abundance of waterbird communities on wetlands can reflect the availability of food in the region generally. For example herbivorous species can be more common in rice-growing areas and fish-eating birds can be abundant around fish farms (Kingsford and Norman 2002). Even temporarily flooded grasslands (1100 ha) can constitute a very important feeding area by supporting large flocks of waterbird species irrespective of breeding (Paillisson et al. 2002). Drying of wetlands Whilst flooding can increase habitat and post-flood drying plays a very important role in concentrating prey in drying wetlands (Dorfman et al. 2001), the importance of a preceding dry phase is still not well understood in Australia and the drying up of water bodies is either ignored or regarded as detrimental to waterbirds (Crome 1988). The drying and filling of temporary wetlands contributes to habitat variability, which can be a key component of the success of some species, such as Black-necked Stork (Dorfman et al. 2001). After extensive inland flooding there are inevitable dry periods that will eventually force waterbirds to move (Kingsford and Norman 2002). But this drying phase before the next inundation is an important part of the long-term ecological processes for the area, for example the need for swamps to dry to provide duck breeding habitat has been well known overseas (Crome 1988). In Booligal (NSW), most species bred best after a flood on a previously dried out basin (Crome 1988) rather than semi permanent water bodies. In the dry period, grasses and other dry land vegetation can grow in the basins and when re-flooded these rich organic substrates and the decaying flooded vegetation provide resources for rapidly developing populations of detritivores such as chironomids (Crome 1988). This is quickly followed by development of a complex wetland flora and a diverse invertebrate population that can eventually becomes dominated by fish (Crome 1988). 62 Before the water completely recedes, the drying period creates new shallow wading areas that can provide new feeding opportunities for many waterbird species. (Kingsford et al. 1999). Fish die in phenomenal numbers as wetlands dry back (Kingsford et al. 1999) and concentrated fish populations can attract large numbers of fish-feeding birds (Whitfield and Taylor 2009). But waterbird numbers can decline rapidly when the increased predation reduced the populations of fish faster than is sustainable (Whitfield and Taylor 2009) After the water has receded waterbird diet may even switch from herbivory to insectivory or they may move from fresh to saline areas, also changing their diet (Kingsford and Norman 2002). The types of birds present can change dramatically during the drying period. In a longitudinal study of a major flood event in the Cooper Creek system Kingsford et al. (1999) found that ducks were almost exclusively dominant pre-flood, numbers of all birds dropped during the flood, herbivores were the most common during the majority of the drying phase, but their abundance fell at least a year before the lake was dry. In contrast, piscivore populations were low pre flood and then rose rapidly and immediately post flood were the most abundant foraging group (Kingsford et al. 1999). As the number of piscivores and (herbivorous) ducks declined dramatically during the drying phase, the abundance of waders reached a maximum (Kingsford et al. 1999). Waterbird population changes for herbivores can be attributed to the changes in abundance and diversity of non-emergent macrophytes caused by altered water regimes in the littoral zone of floodplain lakes (Lesley 2001). A1.2 There’s lots we need to consider before using waterbirds as indicators Little is known of Australian Water birds Although waterbirds are reasonably well studied compared to many other animals, there are many unknowns (Kingsford and Lee 2007). Knowledge of their ecology remains poor for many species, particularly cryptic and rare species, and is only moderate to good for hunted species (Kingsford and Norman 2002). 63 Whilst reduced flooding is perceived as detrimental in general, for many Australian waterbird species, little is known about the impact of reduced altered hydrological regimes on breeding (Kingsford and Johnson 1998). For example, the (long-term) difference in impact of a few small breeding events, compared to an occasional large breeding event is unknown (Kingsford and Johnson 1998). Australia’s arid environment and river systems are inherently variable and we have restricted knowledge of waterbird movements and use of different wetlands in general (Kingsford and Lee 2007) and migration patterns for Australian water birds is relatively limited or unclear (Chambers 2008). Wetland Types Wetlands vary from inundated grasslands to marshes and swamps to inundated shrublands and or forests and different bird species tend to be associated with different wetland types (Desgranges et al. 2006). This is probably mostly because of the different habitat and potential feeding resources on offer. Nevertheless the long-term fluctuations between drought and flooding makes applying classifications for an Australian wetlands difficult (Fjeldsa 1985) and it is simpler to concentrate on the bird communities themselves. Furthermore, decreases in hydrological condition from river management results in a change in the distribution and abundance of wetland types on the regional scale (Desgranges et al. 2006). In other words, different types of wetlands are differentially subjected to different rates of loss (Green et al. 2002). This can have serious consequences for water birds but the changes in waterbird communities may be an indicator of the changes. For example, species that nest in wet grasslands are more vulnerable than migrant species to changes in the water regime as extensive spring flooding during the breeding season can delay or prevent waders from breeding (Paillisson et al. 2002). Wetlands show spatial and temporal variation Ephemeral wetland habitat extent vary as a are a result of Australia’s highly variable climate and river-flooding patterns (Kingsford and Norman 2002). As the wetlands and their associated habitat vary enormously, any classification 64 of wetlands must t be flexible enough to reflect the varying conditions (Fjeldsa 1985). This variation is location dependent. Spatial variation in waterbird numbers The presence of many birds at a site is often an indicator of good environmental conditions, however, the presence of only a few birds may not reflect poor environmental conditions because conditions elsewhere may be influencing bird numbers at the monitoring site (Kushlan 1993). In other words, spatial variability must be understood because even looking across Australia as a whole, there are generally always some refugia available even when there are extended dry periods. On the other hand, when there are more frequent flooding events, colonial waterbirds may have many choices of feeding or nesting sites and birds may not be present in all suitable places at a particular time (Kushlan 1993). We do know however that waterbird movements in Australia are dynamic, with habitat, food and breeding requirements generally determining whether waterbirds move, when they do and how far (Kingsford and Norman 2002). Equally, monitoring and bioassessment need to be aware of the timescales over which ecological, hydrological and geomorphological patterns and processes respond (Vaughan et al. 2009). Understanding what constitutes natural population changes because of normal climatic or environmental variation is necessary before anthropogenic stressors can be implicated (Lesley 2001). Numbers or waterbirds vary widely from one year to another and during the breeding season (Reitan and Sandvik 1996). Some of the variability can be taken out by looking only at presence of the waterbirds or only at their breeding. There are relatively few natural areas in Australia where colonial waterbirds breed (Kingsford and Auld 2005) and much of the overall potential breeding area has declined (REF). Even though natural wetland areas are declining, artificial wetlands like irrigation channels and ponds can support many bird species (Rendo´n et al. 2008) and these artificial impoundments can even provide temporary nest sites (Kingsford and Norman 2002). 65 Migration Regular movements are not common in most Australian waterbird species although some, particularly waders, migrate between Northern Hemisphere breeding grounds and non-breeding habitat in Australia (Kingsford and Norman 2002). Rainfall (either in the form of total rainfall or number of raindays) appeared to be associated with the timing of arrival, departure and season length and this relationship is particularly apparent in drier regions (Chambers 2008). Other factors that may contribute to changes in timing of migration include changes to vegetation, changes in temperature, land-use and water-use (particularly in the case of waterbirds) at both the breeding and non-breeding grounds (Chambers 2008). A1.3 Literature Review of Waterbirds as indicators Waterbird indicators – general The basic biology of colonially nesting waterbirds makes them ideal bioindicators (Kingsford and Lee 2007; Kushlan 1993; Lesley 2001). Waterbirds are a major component of the floodplain wetland ecosystems and integrate information from across the entire ecosystem and indicators can be designed to standalone or to complement other indicators (e.g. flow, fish, vegetation) (Kingsford and Lee 2007). Existing long-term data sets have provided good evidence for the value of waterbird data and its ability to reflect ecological changes to a particular site as a result of water resource development (Kingsford and Lee 2007). Kushlan (1993) identified the following potentially appropriate colonial waterbird bioindicators; genotoxicity, mixed function oxidases, metallothionein induction, tissue concentration of contaminants, egg shell quality, other physiological responses, histopathology and teratology, growth, behaviour, reproductive performance, mortality, presence/absence, distribution, and population indices. Many of those potential indicators mentioned above are indicators at the organism level as such more appropriate to non-migratory birds or from more permanent water bodies. Kingsford and Lee (2007) suggested monitoring floodplain health using waterbirds in the Australian context should include species richness, abundance and breeding indicators. This literature 66 review has also identified potential community structure indicators that may be considered. Waterbird Communities Historically, descriptions of waterbird communities generally correspond to functional groups relating different food preferences and where the birds usually forage (Kingsford and Lee 2007). In Australia waterbirds generally belong to one of five community types and the typical habitats for each community or association are characterized mainly by factors associated with the stability or permanency of the wetland (viz feeding conditions?) and with presence or absence of vegetation cover (Fjeldsa 1985). The kind of vegetation and the topography of the wetland appear to influence the avian community only slightly and the habitat associations are probably more related to hydraulics of the system (Fjeldsa 1985). For example, reduced periods with spring water levels under 25 cm was adverse to numerous ground-feeding waterbirds (Ciconiiformes and waders) that require shallow waters.(Paillisson et al. 2002). On the other hand some species such as ducks are less sensitive to water level fluctuations (Paillisson et al. 2002). Similarly, it is not uncommon for natural wetlands have a higher ratio of migratory to residential species than artificial wetlands (Bellio et al. 2009). Differences in the number and relative abundance of colonial wading bird species between Florida and Venezuela appear to reflect differing hydrological conditions in two otherwise similar ecosystems (Kushlan 1993) Waterbird Community Bioindicators Because of the sensitivity of the entire waterbird community to inundation patterns, a number of different indices can be used to track floodplain health using waterbirds (Kingsford and Lee 2007). The number of waterbirds and the composition of waterbird communities can reflect changes in water quality or changes to flow regime (Kingsford and Lee 2007). These changes can be monitored at the community level using include total abundance, species richness, functional groups, community composition, presence/abundance of particular species (threatened or migratory), abundance of breeding birds species richness of breeding birds(Kingsford and Lee 2007). Changes in 67 ecosystem functioning should alter patterns of colonial waterbird community structure over time (Kushlan 1993). Waterbird Population Bioindicators The presence (and persistence) of a colonial waterbird species at a site indicates that the ecosystem is suitable for it at some level and therefore is an appropriate bioindicators of those conditions that the bird requires (Kushlan 1993). If the species present are of a high nature conservation value then this can add value to the biological integrity and ecological value of a wetland (Paillisson et al. 2002). Colonial waterbird abundance or population data have the potential to provide more information than presence/absence data alone however (Kushlan 1993). Reproductive performance can be an early indication of population effects that appear later (Kushlan 1993) and basic population parameters such as birth rates are among the most appropriate variables to use as bioindicators (Lesley 2001). The timeframe for making assessments using population indicators needs to take into account the reproductive biology of the species considered. For example, some species have an approximate mean wild survival of 8–10 years with a potential lifespan of 20–25 years (Lesley 2001). Treating it as a closed system (i.e. ignoring migration), this would mean a flood one year in 7 would sustain the population perpetually and even a one flood year in 20 may suffice albeit at lower numbers. Therefore long-term monitoring of population numbers may have value as an indicator of wetland health. These numbers could change between species and the relative abundance of species could indicate the inundation history for a closed system. But these differences emerge over long periods and it is likely that concerns over a decrease of an individual colonial waterbird species would overshadow concern for changes in community indices (Kushlan 1993). Bird mortality could be an important indicator and has often been recognized as a signal of broader environmental problems (Kushlan 1993). Although death of a single bird provides little information, Kushlan (1993) suggests that massive or repeated instances of mortality can be considered to be an assay, 68 analogous to typical toxicology bioassay. This is misleading if one wanted to compare regions or wetlands because one cannot generate an assessment unless the organisms (waterbirds) are exposed to each region or wetland. In other words, if birds are absent for whatever reason then they cannot be used to assess that area. We do know that hydrological stress factor is probably the most critical factor affecting long-term stability or persistence of waterbird breeding within the forest (Lesley 2001). Without breeding or migration, some species can become locally absent but this may also be a natural event. It is recommended that the absence of waterbirds could be considered as potential warning signals of degradation that should lead to more direct measurements (Mattsson and Cooper 2006), (e.g. hydrological indicators, macrobenthos monitoring, catchment-scale land use, etc). Richness type indicators In some environments the number of species of waterbirds present is related to the number of waterbirds present (MDBC 2008). On other occasions however the number of species present may be a representation of the health of the ecosystem. For example, the number of duck species can show positive trend with increasing water level (Reitan and Sandvik 1996), and freshwater wetlands can support more species than saline ones (Chambers 2008). On the other hand, whilst the presence of many birds at a site is often an indicator of good environmental conditions, however, the presence of only a few birds may not reflect poor environmental conditions because conditions elsewhere may be influencing bird numbers at the monitoring site (Kushlan 1993). Breeding type indicators Waterbird population indicators will probably require one or two generations to turnover to detect changes (Lesley 2001) and some species may not breed successfully for decades (Kingsford and Norman 2002). A simpler measure may be to monitor breeding events. Some waterbirds have site fidelity for breeding and so monitoring of specific sites could be an option (Kingsford and Lee 2007). Of course this again raises the issue of not being able to monitor 69 the whole continent equally, rather only sites where breeding is known to occur. The types of indicators would be related to commencement of breeding, number and types of species nesting, nest abandonment/desertion events, fledgling success and the sequence of species that breed. There are documented cases of the sequence of triggers for breeding for different species. In south western Queensland, pelican breeding probably coincided with high levels of fish populations while Black Swans nested later as the water cleared and aquatic macrophytes became abundant (Kingsford and Norman 2002). Spatial and Temporal monitoring Australia experiences great spatial and temporal heterogeneity of wetlands because of continuing long- and short-term oscillations between flooding and drought (Fjeldsa 1985). Consequently there is spatial and temporal variability in the availability of Australian wetland habitat and available food resources, which are probably the most important factors determining the abundance of waterbirds (Kingsford and Norman 2002). The considerable spatial and temporal variation in cormorant abundance on semi-permanent wetlands is thought to reflect food availability (Kingsford and Norman 2002). It is therefore not surprising that waterbird abundance in Australia is generally unpredictable and interpretation of such data can be difficult (Kingsford and Norman 2002). Nevertheless, if appropriate indices are calculated and maintain trends over time and space, the underlying population changes should be inferable (Kushlan 1993). In other words, annual monitoring provides only broad information and requires long periods of data to ensure that the stochastic variation can be separated from anthropogenic impacts (Kingsford and Lee 2007). For example, when periods of drought and no egret breeding in the northern half of the Murray–Darling Basin corresponded with floods in the southern half, egret breeding numbers continued to decline in the southern half (Kingsford and Johnson 1998; Lesley 2001). This suggests that immigration is not a mechanism for colony reformation in the Barmah-Millewa 70 forest. (Lesley 2001) and gives an example of how large spatial and temporal scale data sets are very important to interpret local scale changes. Some waterbirds use the same or different wetlands at different times (Kingsford and Norman 2002). This could make investigations of specific distributions difficult (Kingsford and Norman 2002) but could also make birds ideal candidates for assessing wetland condition from a landscape perspective (Veselka IV et al. 2009). Sometimes the variability is predictable and this could be put to use when developing indicators. For example, many tropical waterbirds are driven by the seasonal wet and dry: they spread out onto inundated floodplains during the wet season and then retreat to remnant wetlands in the dry season (Kingsford and Norman 2002). The presence of any particular waterbird community may be as much a reflection of factors operating outside the catchment or site as factors intrinsic to the site itself and so relevant to floodplain condition.(Kingsford and Lee 2007). These factors may act at local, regional or continental scales (Kingsford and Norman 2002). For example, presence of any species may depend on having a regional waterbird population pool available (Kushlan 1993). Waterbird distribution and abundance in one part of the MDB may be affected by flows elsewhere in the Basin (Kingsford and Lee 2007). This is well documented as the boom and bust cycle of Australian dryland river systems (Kingsford et al. 1999). Functional indicators Waterbirds can be allocated to different functional groups according to say nesting requirements or feeding types (e.g. Hughes et al. 2009; Kingsford and Thomas 2004). Changes in nesting numbers of birds from a particular feeding functional group can suggest the effect of a common stressor related to food abundance or availability (Kushlan 1993). Kingsford and Thomas (2004) attributed declines across all feeding functional groups: piscivores (82%), herbivores (87%), ducks and small grebe species (90%), large wading birds (91%), and small wading birds (95%), to similar levels of decline in the aquatic biota that formed their food base. 71 Breeding indicators The breeding of colonial waterbirds is a potentially useful measure for measuring environmental flows because waterbird breeding is highly responsive to high flows and flooding (Kingsford and Auld 2005). Yet not all species respond in the same way, as Kingsford and Auld (2005) found no significant relationship between breeding of Cattle and Little Egrets and river flows. In general, poor reproductive performance is due to some aspect of the environment creating stress and, as a result, can be used as a bioindicator of general environmental stress (Kushlan 1993). Because waterbirds are top order consumers, poor reproductive performance can signal long-term environmental change related to diminished ecosystem productivity at lower tropic levels (Lesley 2001). When habitat or food availability is low there is a reduction in the number and quality of chicks produced (Kushlan 1993). Colonially-nesting waterbirds develop traditional attachments to nest sites that provide reliable nesting and foraging habitats but local breeding populations can expire from prolonged dry periods (Lesley 2001). Reinstating a suitable hydrological regime under these circumstances, may prove an ineffective recovery strategy (Lesley 2001). Irrigation developments in the Cooper Creek catchment to divert water from the river and decrease the frequency and flooding of wetlands of the Lower Cooper were predicted to result in fewer feeding areas and less breeding opportunities for waterbirds (Kingsford et al. 1999). Consequently, boom periods would be shorter and bust periods longer (Kingsford et al. 1999). Growth Growth and condition are commonly used bioindicators in various animals (Kushlan 1993). Using a waterbird example, a link has been inferred between growth of young herons and water conditions in their feeding habitat (Kushlan 1993). 72 Waterbirds abandon/desert nests/stranding Inter and intra annual variations in depths changes wetland areas which can lead to an increased probability of nests being stranded particularly for water birds that nest near the surface (Desgranges et al. 2006). When a nest becomes stranded the birds face the inevitable energetic conflict between moving to another habitat or remaining where decreasing resources may become abundant (Kingsford and Norman 2002). Australian Pelicans also sometimes stay and die (Kingsford and Norman 2002) but many species desert or abandon the nest and their offspring. Ibis abandoned their almost fledged chicks on Lake Altiboulka, following rapid recession of a natural flood (Kingsford and Norman 2002). Nest desertion is a result of inadequate flows (Kingsford and Lee 2007) and has been directly attributable to periods when water levels dropped rapidly from pumping upstream (Kingsford and Auld 2005). The rate of fall rather than the fall itself was associated with abandoned breeding at the Algeboia Plain (Lesley 2001). Although proximate factors for such abandonment are probably associated with falling water levels, ultimate factors are probably related to food availability (Kingsford and Norman 2002). Positives for using waterbirds There are a large number of waterbird species found in Australia (81 in the Murray–Darling Basin alone) leading to a wide range of potential responses (Kingsford and Lee 2007). Among the positives for using waterbirds are; Waterbirds can track ecosystem health at the landscape scale, including key wetlands and entire catchments (Kingsford and Lee 2007) The structure of waterbird communities and functional distribution patterns is strongly associated with habitat quality, changes in landuse and physical disturbance to the bank profile (Hughes et al. 2009) Waterbirds are predominantly at the top of the food chain and so they can track changes in other indicators (e.g. invertebrates, vegetation, fish) providing an additional indirect measure of these indicators (Kingsford and Lee 2007) 73 Trained amateurs can identify bird species through passive observation or playback recordings of focal species (Mattsson and Cooper 2006), this is considerably less sampling effort than for fish or macroinvertebrates Using stream-dependent birds as an early warning signal for degradation of stream biotic integrity could improve the efficacy of catchment monitoring programmes in detecting and identifying perturbations within the catchment. (Mattsson and Cooper 2006) Population indices for colonial wading birds in the Everglades have been shown to change in relation to changes in wetland hydrology (Kushlan 1993) The limited number of sites used by colonial waterbirds and their geographic restriction make them particularly vulnerable to anthropogenic impacts (Kingsford and Auld 2005) There is good evidence that waterbird communities have already responded to changing flooding regimes in the Murray–Darling Basin (Kingsford and Lee 2007) Negatives for using waterbirds There are, however, major difficulties in using colonial waterbird population indices as bioindicators of ecosystem change (Kushlan 1993). These include; Presence does not mean that all aspects of a bird's needs are met; a bird may be present but not reproducing. (Kushlan 1993) Mobility of the species (Kushlan 1993) Lakes may temporarily have a much wider selection of species than normal because of drought somewhere else (Fjeldsa 1985) Breeding populations of colonial waterbirds are known to rebound after natural and human induced reductions (Lesley 2001) Colonial waterbirds are large in size, need wide ranging habits, are difficulty to approach and capture, and migrate (Kushlan 1993) Waterbirds can concentrate in artificial wetlands when natural wetlands are dry (Rendo´n et al. 2008) Inherent variation in Australia’s climate may make any long-term, systematic impact difficult to discern above shorter-term, background 74 variation (Kingsford and Norman 2002). Can boom and bust cycles be quantified and converted to an indicator water related stress? Waterbird communities are effective indicators of the ecological integrity of wider river landscape (Hughes et al. 2009) – it may be difficult to partition out the effects of water related stress from other effects such a habitat fragmentation, pollution, etc High natural variability means that distribution changes may prove hard to distinguish from short-term fluctuations (Kushlan 1993) Factors other than flow patterns may be implicated in the decline of waterbirds The proliferation of Cyprinus carpio and grazing by domestic stock are examples of confounding environmental stressors (Lesley 2001) When a downward trend of a population index is detected, it may not be possible to know what is causing the trend without doing a causality study (Kushlan 1993) Other factors such as weather and contaminants also can affect nesting success. (Kushlan 1993) Populations trends can be caused by environmental changes (Kushlan 1993). The many factors affecting population density at a particular site suggest that it may be difficult to relate population data to overall habitat value (Kushlan 1993) The absence of a species at the time of sampling does not prove that ecosystem conditions are unsuitable for it (Kushlan 1993) Wetland loss and development of water resources are formidable issues, closely tied to future economic development in Australia (Kingsford and Norman 2002) Caveat. Waterbirds numbers are declining anyway Waterbirds are declining around the world (Bellio et al. 2009; Rendo´n et al. 2008). Unless policies protecting rivers are implemented, habitats for waterbirds will continue to disappear (Kingsford and Norman 2002) and waterbird numbers and local diversity will continue to decline. The declines are well documented for some regions. For example, The number of 75 waterbird species in the Lower Murrumbidgee has declined significantly by 21% since development (Kingsford and Thomas 2004). The large colony sizes in the Macquarie marshes (of more than 100,000 nests estimated during the floods of the 1950s) are unlikely to occur again (Kingsford and Johnson 1998). Leslie (2001) identified evidence of declining abundance and diversity of colonially-nesting waterbirds within all four waterbird functional guilds in the Barmah-Milewa Forest. 76 Appendix 2 Literature Review of Vegetation Ecology relevant to water scarcity Physical habitat in streams is a major determinant of in-stream biotic composition (Bunn and Arthington 2002) and a major component of habitat is in channel vegetation. The aquatic life and process in the channel are adapted to this flow regime (Bunn and Arthington 2002). Outside the main channel, floods, dry periods, and seasonal flows are all part of the flow regime and are the equivalent on the floodplains and for floodplain vegetation as the river flow regime is for in channel processes (Roberts and Marston 2000). Vegetation needs water for survival and regeneration phases and the water regime for each phase may be different (Roberts and Marston 2000). Hence different types of floodplain and or wetland vegetation are adapted to and require differences in components of flow regime including; o Magnitude of flood o Duration of flooding o Rate of flood ascension/recession o Frequency of floods o Seasonality (Roberts 2004; Roberts and Marston 2000) Hence disruption to natural flow regimes should be observable in changes to channel or floodplain vegetation characteristics. However, vegetation on the margins of inundation zones can also use rainfall stored in the soil is an additional possible source (Thorburn et al. 1994). The spatially and temporally complicated nature of these environments means the adaptive strategies used by plants can be complex (Thorburn et al. 1994). For this theme the challenge will be to isolate water scarcity effects from other effects. What happens to vegetation when it is water stressed? Plants can use rainfall, surface water or groundwater. All these may be available in flooded conditions in wetlands or on floodplains, whereas in 77 drought periods groundwater is their only source of water when surface water bodies have dried up and soil water reserves have been depleted (Thorburn et al. 1994). In marshy areas, rainfall and groundwater are the two primary sources of water for plants (Thorburn et al. 1994). Overall, the effects of changes in the inundation regime on plants depends on whether a plant has a narrow or wide range of environmental tolerances and whether the species is resistant (i.e. has capacity to withstand stress) or resilient (has the capacity to recover) (Bunn and Arthington 2002). The frequency and intensity of exposure to hydrological stress by floodplain vegetation are increased by river flow regulation(Jensen et al. 2008). River regulation is associated with many changes in flow apart from less water availability (Poff et al. 2009; Poff and Zimmerman 2010). A thorough literature review of riparian ecological responses to change in flow regimes was conducted by Poff and Zimmerman (2010, Table A2.1). Table A2.1: Responses of riparian zones vegetation to altered flow regimes from river regulation (source: Poff and Zimmerman 2010) Flow component Primary flow alteration Riparian Ecological Response Magnitude loss of peak flows Altered recruitment, failure of seedling establishment Terrestrialisation of flora Increased success of non-native Lower species richness Vegetation encroachment into channels Increased riparian cover Frequency Decreased frequency of peak flows Shift in community composition Reductions in species richness Increase in wood production Duration Decreased duration of floodplain inundation Reduced growth rate or mortality Altered assemblages Terrestrialisation or desertification of species composition Reduced area of riparian plant or forest cover Timing Loss of seasonal flow peaks Reduced riparian plant recruitment Invasion of exotic riparian plant species Reduced plant growth and increased mortality Reduction in species richness and plant cover Rate of change Increased variability Decreased germination survival and growth of plants 78 Dry climate floodplain vegetation communities are adapted to but prone to moisture stress (Roberts 2004) and show varying responses including include slower growth, dieback or mortality when there is a shortage of available moisture (Jensen et al. 2008). The variation in responses can be because of differences in species and individual site circumstances but the general effect of moisture stress response in trees is; Vigour for existing mature trees o fewer and shorter periods of growth conversely more and longer periods of little no growth or water or heat stress Regeneration o fewer opportunities to flower, set seed, germinate or for seedling establishment (Roberts 2004) And the typical responses of understorey and wetland plants; flood events not long enough to complete life cycle and set seeds flood events not long enough to re-establish carbohydrate reserves in perennating organs depletion of seed banks through decay, ageing and predation due to infrequent flooding attrition of perennating organs (rhizomes, rubbers) due to fewer and shorter opportunities for growth (Roberts 2004) Scale of changes in vegetation associated with hydrological stress Scale of hours (Merritt et al. 2010) Somatal conductance, transpiration, net carbon assimilation, leaf internal CO2 concentration, carbon isotope discrimination (an index of time –integrated carbon concentration and water use efficiency) and xylem water potential can change as plants increase their water use efficiency or become stressed in response to anoxia, water availability or changes in atmospheric conditions. Such measurements have led to the development of thresholds of groundwater depletion for some riparian species through measuring the onset and consequences of chronic water stress 79 Scale of hours-weeks (Merritt et al. 2010) Wilting, chlorosis and discoloration of leaves, abscission, leaf death and reduction in canopy volume can collectively express negative, short to long term (hours-weeks) response to reduced water availability. Extreme water stress may result in xylem cavitation and branch dieback in trees and shrubs (‘drought pruning’), which can relieve overall water stress in the individual by reducing leaf area. Severe moisture stress sometimes results in complete branch or tree dieback allowing reduced stress in surviving branches and individuals within a riparian forest stand. Long-term responses (Jensen et al. 2008) Slower growth and dieback or mortality will become more common. Plants may continue to produce seed under stress, and germination may occur opportunistically in response to local rainfall, but the seedlings are insecure until they have developed ‘sinker’ roots to free them of dependence on nearsurface soil moisture. Salinity and the interaction between groundwater and surface water Salt accumulates in floodplain soils as a result of capillary rise of water transporting the natural salts to the surface (Overton et al. 2006). Shallow groundwater is drawn up and either evaporated at the surface, or used by the trees (Overton et al. 2006) and river regulation can cause water tables to rise closer to the surface, increasing salt accumulation rates in floodplain soils (Holland et al. 2009). The salt is left behind in the upper soil layers where it slowly accumulates where historically it would have been flushed by flooding every few years (Holland et al. 2009; Jolly et al. 2008; Overton et al. 2006). Reductions in the frequency, duration and extent of flooding mean that the leaching of salt from the plant root zone is reduced, causing a reduction in soil water availability and riparian vegetation health (Holland et al. 2009). Alternatively, in areas where rivers lose water to the groundwater, reductions in the frequency, duration and extent of flooding can result in lowering of water tables beneath floodplains (Holland et al. 2009). If the groundwater is 80 relatively fresh, then lowering of water tables results in a reduction of water availability (Holland et al. 2009). This water scarcity related stress can potentially be detected by changes in the vegetation. For example, persistence of submergent species likeVallisneria australis and the woody shrub Melaleuca ericifolia was compromised by increased salinity (Salter et al. 2008). Increased salinity was associated with areas of non-tree vegetation in poor condition on the Chowilla floodplain, and an increasing occurrence of saltbush species (Overton et al. 2006). Wetlands can slowly change from ephemeral wetland environments to floodplain halophytic communities in such conditions (Overton et al. 2006). The two main species of Eucalypts associated with floodplains in the Murray– Darling Basin are River Red Gum (Eucalyptus camaldulensis) and Black Box (E. largiflorens). River red gum has lower tolerance to salinity than black box and generally most black box exists on the higher parts of the floodplain where depth to groundwater is rarely less than 2m (Overton et al. 2006). The differences in salinity tolerance means that large scale changes to flood regimes and subsequent lack of salt flushing means that Black box can become more favoured in some riparian zones. Potential Indicators Plant community attributes such as richness, diversity, cover, growth, productivity, community composition and biomass can be linked to the hydrologic attributes of rivers and may respond in predictable ways to specific hydrologic alterations (Merritt et al. 2010). Plant associations or dominant cover types show strong affinities for specific hydrologic attributes such as inundation duration and depth to ground water (Merritt et al. 2010). A recent study from Africa showed that indicators can be derived for woody plant variables response to desiccation-driven change (water-table lowering and increased salinisation) (Ringrose et al. 2007). Distinct changes in tree and shrub height, plant density and species richness as well as changes to abundance of brackish ground-water-tolerant and dryland species were 81 related to water scarcity (Ringrose et al. 2007). The next section looks through some potential indicators in an Australian context. Terrestrialisation Differences in tolerances to stressors means that the make up of communities can change through time. The process of changing to drier conditions is sometimes referred to in floodplain vegetation ecology as Terrestrialisation, meaning that overall the floodplain is beginning to support species more typical of terrestrial environments and habitats (Roberts 2004). Plant communities undergoing Terrestrialisation can be expected to progress through stages such as; loss of vigour, (initially in sensitive perennial species) loss of species shift in structural characteristics such as cover and number of strata gradual establishment of species better adapted to drier conditions (Roberts 2004) Detectable changes in vegetation characteristics therefore include species richness, number of strata, cover of dominant trees and groundstorey species (Roberts 2004). Terrestrialisation is not officially defined and at this stage is only applied in an anecdotal sense (Roberts 2004). However this type of response is the move towards saltbush species on the Chowilla floodplain described by Overton et. al (2006). The response of large long-lived species like River red gum may be much slower than other species. Whilst the total area of River Red Gum forests and woodlands in Yanga National Park remained over a sampling period of 40 years there were significant changes in the areas of River Red Gum understorey communities (Wen et al. 2009). Spike-rush ground cover was associated with high soil moisture after a decade of above normal flooding but drying saw a change to dominance by chenopod shrubs or bare ground understorey (Wen et al. 2009). Similarly, invasions by more droughttolerant species such as chenopod (e.g. Nitre Goosefoot (Chenopodium 82 nitrariaceum) and shrubby species such as Lignum (Muehlenbeckia florulenta) occurred in the Murrumbidgee catchment after water resource development (Kingsford and Thomas 2004; Wen et al. 2009). Lignum is a common and widely distributed shrub of Australia's desert floodplains and is very tolerant to flooding and drying (Capon et al. 2009). Lignum tolerates drying by reducing leaf area ratios and recruitment is a likely response to surface water hydrology (Capon et al. 2009). Vegetation responses to water scarcity include not just less water but changes in inundation history like less frequent flooding or reduced area of flooding. The drawdown of Lake Mokoan allowed for documented responses of vegetation to a controlled change in inundation from wetter and younger to older and drier (Roberts and Hale 2007). There was an increase in species richness, in the number and the proportion of terrestrial species, the number and relative proportion of perennial species, a decrease in nativeness (% of species native), and in the proportion of species that were mainly water dispersed (Roberts and Hale 2007). Terrestrialisation at Lake Mokoan was a gradual process, with no abrupt ecological changes, -The oldest and driest quadrats, were not significantly different from each other in terms of floristic composition (Roberts and Hale 2007). Floristic composition was virtually defined after four years without inundation and that timeframe is therefore an ideal point to review vegetation status (Roberts and Hale 2007). Species richness The response of species richness to drying is certainly time dependant. For example, species richness of seeds in dry-stored wetland sediments decreased by approximately 50%, to approximately 25 species over just 9 years (Roberts 2004). Therefore this type of indicator (seed bank species richness) would need to be consider species resilience times. It is suggested that River red gums require inundation once in every 4 years whilst Black box only 1 in 12 years for persistence (Roberts 2004). Therefore any richness 83 indicator can be value added in a water scarcity context by taking into account the composition of the species present (Mathews 2009). Vegetation form Hydrological stress may be evidenced by changes in the 3 dimensional structure or missing layers from the strata of the riparian vegetation. Roberts (2004) cites a paper suggesting that upper stratus should have at least 50% old mature or mature but not emergent stratum. But there is little quantified research available in this topic. Similarly, native and alien cover needs further research, but an index using non-native cover of lower strata <1.5 m tall has been used and there may be an apparent inverse relationship between flooding frequency and number of introduced species in the Barmah Forest (Roberts 2004). These types of anecdotal relationships are often open to confounding and natural variation so further investigative research would be required for them to become viable indicators. Whilst increased dominance by natives may indicate the return to conditions for which the natives are adapted, intermittency is not unusual in desert riparian systems and therefore dry conditions are not necessarily expected to favour non-native herbaceous species over natives (Katz et al. 2009). Thus, dominance by native herbs per se may not be a measure of hydrologic restoration in this system as non-native dominance may also reflect general levels of disturbance such as the degree of present or recent agricultural activity (i.e. livestock grazing, irrigated crops (Katz et al. 2009). Perennial-flow reference sites had higher herbaceous cover, higher species richness, lower weighted wetland indicator scores, and higher relative cover of hydric perennials and hydric annuals than non-perennial sites (Katz et al. 2009). In contrast, non-perennial sites had higher relative cover of mesic perennials and xeric annuals (Katz et al. 2009). All of these are useful metrics for assessing riparian condition (Katz et al. 2009) Condition of floodplain vegetation The condition of floodplain vegetation can be considered by vigour, population sustainability, and ecological integrity. Specific attributes relative to these 84 attributes can be measured or estimated, and applied to dominant trees or to the whole community, as required (Roberts 2004). Vigour Water availability along an environmental gradient leads to a decrease in forest productivity and leaf area index over that gradient (Horner et al. 2009). Thus changes in vigour can be a result of a natural spatial gradient when distance from the river channel relates to decreasing water availability in some forest stands (Cunningham et al. 2007b; Wen et al. 2009). Visual assessment of canopy condition on the Chowilla floodplain after reports of increased dieback found the few live trees of both River Red Gum and Black Box were trees mainly in patches beside the River Murray (Roberts 2004). A study of tree health from Renmark to Walker Flat where over 80% of trees showed some stress, found stressed trees were typically associated with dry creeks and lagoons, or where saline ground water flowed into the creeks (Roberts 2004). Similarly, temporal changes in site productivity due to decreasing water availability led to reductions in leaf area and stand density for long-lived organisms such as trees (Horner et al. 2009). Vigour is often surrogated by measuring canopy cover or crown condition. Crown condition is known to improve in River Red Gums with frequent flooding and is apparently closely linked with the hydrological regime within the floodplain (Wen et al. 2009). Dieback Overall levels of dieback remain as a potential general indicator of water scarcity related stress, but there are other causes of dieback and within stands there can be considerable variation in the level of dieback. Drought – related dieback could cause higher mortality in smaller trees as larger trees have access to groundwater, or higher mortality in larger tress because of reduced vigour, making vigour and stand dynamics in combination a potential indicator. However, a recent study of E. camaldulensis (in a stand showing considerable dieback) found different-sized trees had similar probabilities of being alive (Cunningham et al. 2009). It is possible that the cause of water stress may differ among different-sized trees, with small trees responding to 85 low surface soil moisture while large trees are affected by lower availability of groundwater (Cunningham et al. 2009). Either way. A lot more research needs to be done before stand dieback differentiation could be used as an indicator. Dieback in the Murray river is related to longitude, and this may be a reflection of decreased water availability downstream which may in turn be affected by decreasing rainfall or increased rive regulation (Cunningham et al. 2009). Nevertheless the high levels of River red gum dieback in the lower MDB is likely to be associated with recent droughts and increased reliability on groundwater (Cunningham et al. 2009). Dieback patchiness may be because of differences in water availability on small scales such as minor differences in topography (affecting the distribution of flood waters), different water holding capacities of soil types and differences in the depth and salinity of groundwater (Cunningham et al. 2009). Community structure Plant communities can be linked to hydrologic attributes of rivers and may respond in predictable ways to specific hydrologic alterations such as inundation, duration and depth to ground water (Merritt et al. 2010). Vegetation characteristics (by indicator) Attributes used to characterize vegetation include biomass, vegetation volume, growth rates and stand physical structure. Yet the response of these attributes to river flow or hydrological alteration are often very river and sitespecific, limiting transferability of relationships to other rivers even in the same hydroclimatic regions (Merritt et al. 2010) Suggested Indicators The following indicators have been used in Australian studies looking at the health of riparian or floodplain vegetation (from Roberts 2004); ecological condition (expressed as hectares of dead trees) non-native species (% of all species) canopy condition (arbitrary % alive indicates healthy) 86 regeneration density (recorded as the number of juveniles and seedlings per hectare) number of regenerating trees in size classes (classes chosen to indicate more recent or not so recent) All the above are measured from in situ surveys, but it is interesting to note that the amount of severely degraded vegetation can be detected from aerial photography and this allows analysis of longitudinal patterns back to the 1980’s (Roberts 2004). A more recent study tried to relate overall stand condition in E. camaldulensis to water related stress and investigated which indicators that could be used in a quantitative assessment of E. camaldulensis stand condition (Cunningham et al. 2007b). Interestingly there was little evidence of differences in physiological performance in trees among goodand poor-condition (Cunningham et al. 2007b). This suggests that the death of many trees and a resultant reduction in tree density of the stand results in a level where soil moisture is adequate for the few living trees (Cunningham et al. 2007b). Hence measures on indivual tress may mask the overall stand condition and remotely sensed data can be used to estimate stand condition across large areas (Cunningham et al. 2007b). Seed production Seed volumes released from unhealthy River Red Gums or Black box can be significantly less than volumes released from healthy trees, but the differences may be related to seasonality more than stress (Jensen et al. 2008). As such seed production makes a poor potential indicator until more is known of natural variation. Remote Sensing Remote sensing offers the capability to monitor a wide range of landscape biophysical properties relevant to management and policy, including plant (crop, forest, natural ecosystem) growth, yield and biomass; soil moisture; water loss by evaporation; flood areas; fire hotspots and fire scars; sunlight amount; and some soil properties (McVicar et al. 2003). Information on these variables can be obtained for the past, the present and the future. Remote 87 sensing complements, rather than competes with, in-situ monitoring systems and modelling (Cunningham et al. 2007a; McVicar et al. 2003). Maps of green, actively growing vegetation, and its departure from normal conditions for the time of year can be readily generated using remote sensed data (McVicar et al. 2003) and Plant Area Index (PAI) and vigour for River red gum stands were successfully predicted using just GIS layers of environmental variables (Cunningham et al. 2007a). The best predictive model for PAI used 6 and Vigour used 4 variables all derived from remote sensed data (Cunningham et al. 2007a). The models were able to choose from long-term (9 years) variance or mean values or the most recent years value and from SPOT or LandSat TM data (Cunningham et al. 2007a). The commonly used Normalised Difference Vegetation Index (NDVI) calculates the photosynthetic capacity of vegetation based on the difference in reflectance of the surface to visible (red) and near-infrared light (McVicar et al. 2003). This quantity has been shown to be a useful (albeit not perfect) index of the proportion of the surface covered by green vegetation, and hence of the vigour of the vegetation (McVicar et al. 2003). NDVI was the single most important variable for predicting PAI in the Murray River floodplains, although its performance as a lone predictor is not given (Cunningham et al. 2007a). In a similar study Cunningham et al. (2007b) found very strong relationships between NDVI and several condition variables including PAI and crown vigour (Table A2.3). The strong relationship between vegetation vigour of mixed E. camaldulensis, E. coolibah (coolabah) and M. forentula and inundation history were modelled in the Condamine-Balonne region using NDVI by Sims and Thoms (2000). They used five classifications of vegetation growth vigour (incorporated variation from the long-term mean) and the top four categories reflected inundation history well. Interestingly however, very low growth vigour was common only where flooding occurred more frequently than once per year (Sims and Thoms 2002). This result shows that site specific information is always needed because of possible interactions with other factors like soil 88 anoxia, disturbance frequency or pixel mixing at the vegetation/water boundary (Sims and Thoms 2002). Table A2.3: Pearson’s correlations (n=12 sites) among the condition variables measured in the field and with the remotely sensed vegetation index NDVI Significant correlations are indicated: *P<0.05. Strong correlations (r>0.75) are indicated by superscript A. (source: Cunningham et al. 2007b). The leaf area index available through MODIS gives reasonable estimates most cover types and land use types in Australia (Hill et al. 2006). Whilst overestimation occurs in some eastern Australian open forests and woodlands, (Hill et al. 2006) a monitoring program that uses historical references would still detect changes through time. Temporal NDVI series have been shown to be an accurate signal of vegetative phenology, which in turn is a fundamental vegetation property and analysis of temporal series of NDVI yields are suitable for regional scale applications (Lobo et al. 1997). Issue of temporal and spatial scales Measures of the physiological characteristics of individual plants may be the most sensitive to short term changes in flow regime, but reveal little about the important ecological consequences of changes in stream flow (Merritt et al. 2010). The choice of measurement at the level of the individual, population or community level or some combination will largely depend upon the vegetation attributes that are deemed important, along with established goals for maintenance and restoration of riparian vegetation (Merritt et al. 2010). NDVI is known to cycle through different seasons naturally, and this may give a summer peak or a spring peak in NDVI. S (Lobo et al. 1997). Hence 89 anomalies are considered to be more valuable than unadjusted raw NDVI scores. Other factors affect vegetation health Riparian taxa are influenced by channel incision, channelisation, increased in floodplain soil salinity and other physical factors so vegetation communities may be responding to flow indirectly through a wide range of physical and biotic factors (Merritt et al. 2010). A number of factors other than groundwater depth and flooding frequency affect the extent of soil salinisation, including the salinity of the groundwater, and soil texture (Overton et al. 2006). Stressors other than flow regime include rising saline groundwater and land clearance and grazing can affect Eucalyptus spp. on the Murray, especially where regeneration is sparse or patchy (Jensen et al. 2006). Because of factors such as climate, soil properties, groundwater, species biogeography, assessments will be floodplain-specific and unlikely to be linear (Roberts 2004). At Lake Mokoan, the quality of the vegetation was linked to time since last disturbance. For example, older and (therefore) less disturbed quadrats had more terrestrial functional types, more non-native species, more species dispersed by animal vectors, and greater species richness (Roberts and Hale 2007). Indicators such as species richness can be non-comparable between different sites or even areas within a site. For example, communities with the highest percentages of non-native species were ‘on higher ground’, or were ‘highly disturbed’ or had an understorey replaced by weak species (Roberts 2004). Conversely, the lowest % of non-native species was for Red gum + spike rush + spiny mud grass open forest community which is wide spread and occurs on low-lying or more frequently-flooded sites (Roberts 2004). Several factors can be responsible for disguising potential patterns using NDVI such as the spatial accuracy of the data, non-vegetation influences on the NDVI, the scale of variation, and the measures for productivity and persistence of productivity (Bader 2000). 90 Exotic veg Lippia Phyla canescens is one of the few significant environmental weeds on lowland floodplains in the Murray–Darling Basin and is a species that persist under dry conditions so should be disadvantaged by flow rehabilitation (Roberts 2004). Issues with using vegetation as an ecological indicator Advantages/strengths 1. There are lots of types/forms of vegetation with different lifehistories/relationships with flow. 2. NDVI and ground truthing may indicate changes a range of temporal scales (i.e. seasonal, internannual) Negatives/weaknesses 1. Temporal and spatial complexity – separating out climate from water extraction stress. 2. The nature of the response of vegetation to hydrological stress. For example: with red gum, the response might be asymptotic, In such a situation, the response is very delayed to the extent of the stress previously occurring, and that pass this point, may have reached a point of no return. So while you can use vegetation as an indicator of stress, you also run the risk of loss of ecological integrity, especially if the relationship between stress and indicator responses, is not known, or is delayed. 91 Appendix 2.1 README FILE FOR MODIS-DERIVED Land Products (Paget and King 2008) CONTENTS -------1) DATA CUSTODIAN 2) ACCESS AND CONDITIONS OF USE 3) CITATION 4) DISCLAIMER, COPYRIGHT AND LICENCE 5) DATA SOURCE 6) DATA INFORMATION 7) DIRECTORY, FILENAME AND DATA FORMATS 8) GEOGRAPHICAL COVERAGE 9) DATA QUALITY 10) VERSION INFORMATION 11) UPDATES 12) REFERENCES 13) CONTACTS 1) DATA CUSTODIAN -------------------------------------------------------CSIRO Marine and Atmospheric Research is the custodian of this dataset. This dataset has been developed by the Time Series Remote Sensing Team, CSIRO Marine and Atmospheric Research. The original data were supplied by the Land Processes Distributed Active Archive Center (LPDAAC), located at the U.S.Geological Survey (USGS) Earth Resources Observation and Science Center (EROS) http://LPDAAC.usgs.gov. 2) ACCESS AND CONDITIONS OF USE -------------------------------------------------------These data are available from: http://www-data.wron.csiro.au/rs/MODIS/LPDAAC Please note the citation and legal information in Sections 3 and 4 below. In order to help us keep track of who is using the data, please do not pass the data on to a third party. Instead, refer them to the web site. In return for free access to these data, we ask that you send us citations and copies of publications arising from work that use these data (Matt.Paget@csiro.au). 92 3) CITATION -------------------------------------------------------When referring to this dataset in publications, please cite both: Paget MJ and King EA (2008) MODIS Land data sets for the Australian region. CSIRO Marine and Atmpospheric Research Internal Report No. 004. http://lpdaac.usgs.gov/citation.asp 4) DISCLAIMER, COPYRIGHT AND LICENCE -------------------------------------------------------Use of these data is subject to the Legal Notice and Disclaimer at http://www.csiro.au/org/LegalNoticeAndDisclaimer.html These data are Copyright, CSIRO, 2008. These data are made available under the conditions of the Creative Commons Attribution-Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/ 5) DATA SOURCE -------------------------------------------------------Data are derived from the MODIS Land Products that are distributed by the LPDAAC as tiles from a global sinusoidal projection. The primary reference for the MODIS land products is http://lpdaac.usgs.gov/modis/dataproducts.asp. Tiles for selected products for Australia have been downloaded and then mosaiced and remapped using the Modis Reprojection Tool (MRT).The products have been split into individual bands to reduce file size and have been reformatted to ensure consistency in SDS and attribute nomenclature. Data from the MODIS instruments on both Terra and Aqua have been used, with spatial resolutions of 250m, 500m and 1km, depending on product.Temporal frequency of the data is daily, 8 days or 16 days, once again, depending on the product, A full explanation of the processing is provided in the report referred to in section 3 (above). 6) DATA INFORMATION -------------------------------------------------------Data are available for a variety of terrestial applications. Products include: 93 Surface reflectance (MOD09Q1, MOD09A1) Land surface temperature and emissivity (MOD11A1, MYD11A1, MOD11A2) Vegetation indicies (MOD13Q1) Thermal anomalies / Fire (MOD14A2) Leaf area index and Fraction of photosynthetically active radiation (MOD15A2) Gross Primary productivity (MOD17A2) Bidirectional reflectance distribution function and albedo (MOD43B1, MOD43B3, MOD43B4, MCD43A1-A4, MCD43B1-B4) The data are identified by the EOS product code. A generic product code is MxDnnyy where, x = 'O' (Terra), 'Y' (Aqua), 'C' (Combined Terra and Aqua). nn = Product number, which defines the type of product. yy = Sub-product code, which may distinguish the spatial and/or temporal resolution. Full detail on these and other products, including the Algorithm Theoretical Basis Document (ATBD), are available from the LPDAAC website. The term "collections" refers to the MODIS land product versioning system. Data products within a collection have been processed with specific algorithm, instrument characterisation and calibration refinements. For many products there are significant changes between collections that reflect a new understanding of physical processes or new ancillary data sets. Therefore caution should be exercised if mixing data from two or more collections. The collection number of a product is identified by a threedigit number (001-005) following the product code. Collection 5 is the current version and was implemented for all data produced from 01 January 2007. The LPDAAC is currently reprocessing the entire data set from launch to 31 December 2006 to collection 5. The majority of the data available here is collection 5, however for some products collection 4 data is retained until the corresponding collection 5 data become available. If collection 6 is released at some future time, that data will most likely be included here. 7) DIRECTORY, FILENAME AND DATA FORMATS ------------------------------------------------------The ftp site has two levels of subdirectories. The first level denotes the product and collection (version) and the second level denotes the epoch. A daily product: 94 MOD11A1.005/ 2007.02.01/ 2007.02.02/ 2007.02.03/ ... An 8-day product: MOD43A1.005/ 2007.01.01/ 2007.01.09/ 2007.01.17 ... A 16-day product: MOD13Q1.005/ 2007.02.18/ 2007.03.05/ 2007.03.21/ ... Filenames have the following format: MXDxxxx.yyyy.ddd.aust.ccc.bNN.label.hdf.gz where, X = xxxx = yyyy = ddd = aust = ccc = bNN = original label = hdf.gz = Spacecraft, 'O'=Terra, 'Y'=Aqua, 'C'=combined (both) Product code Year GMT day number that the time-step begins (Jan,1=001) Designates an Australian regional mosaic Collection number Band number (retains the order of the SDSs from the product) Short descriptive name for the SDS HDF file, gzipped Data are supplied as gzipped (standard) HDF files with one file per band, where the band relates to the order of the SDS's in the LPDAAC product. All of the HDF-EOS metadata are retained as global attributes. In the cases where the band is a byte or bitmap index (e.g, quality flags) an index is supplied as an attribute. The Report (Sec. 3) contains full details on the mosaicing, remapping and reformatting processes. Since May 2008 (processing date rather than epoch), log files relating to the processing have been included with the mosaic data files. Individually the log files are text files. They are provided as gzip-ed tar files. The tar filenames have the following format: logs_yyyymmddThhmmss 95 where, yyyymmdd = Year, month and day of the processing. T = A separator. hhmmss = Hour, minute and second of the processing. The log files consist of: hdf.list = List of input LPDAAC files. remap.par = Parameters for the resample program (MRT). bXX_mosaic.log = Output from the mosaic program (MRT). bXX_remap.log = Output from the resample program (MRT). bXX_reformat.log = Output from the reformatting program. where XX is the band number. Products in the mosaic archive may be regenerated with progressively more complete data over several days as the LPDAAC data pool is populated. The mosiac files will be overwritten with the latest data. The log files, however, will not be overwritten as they are time-stamped with the time of processing. Therefore, the log files act as a record of the processing for each band and for the epoch. 8) GEOGRAPHICAL COVERAGE ------------------------------------------------------Data are unprojected, in geographic decimal degrees, referenced to WGS84. The data are all remapped to the region 10.0 - 45.0 S and 110.0 - 155.0 E. The number of pixels, pixel size and exact spatial extent differ between the products depending on the spatial resolution of the data (e.g. 250, 500 or 1000 m). Precise geolocation information for each of the spatial resolutions is provided in Appendix B of the report (Paget & King, 2008 - see Sec. 3 above). 9) DATA QUALITY ------------------------------------------------------LPDAAC are responsible for the actual data quality and provide quality channels as part of most products. These channels are processed the same as the data channels. The mosiacing, remapping and reformatting processes do not alter either the data or the quality fields. Users should be aware that not all tiles for a particular mosiac necessarily become available from the LPDAAC on the same day. It is therefore possible that the most recent mosaic for any given product will be updated over several days, each time containing progressively more and more actual data within the same (standard) geographic extent. 96 We have had some minor issues with file corruption that have been detected by users unable to un-gzip the mosaic files. If you discover any such files, please report them to us (see contacts below). 10) VERSION INFORMATION -------------------------------------------------------------Please see section 6 with reference to 'collections'. 11) UPDATES -------------------------------------------------------------The mosiac collection is automatically updated daily. In practice this means that the archive is updated within 24 hours of new data being released on the LPDAAC data pool. The documentation (papers and reports, and possibly this README file) will mostly reflect the state of the data set at the time of its initial release and may not be updated in synchrony with the data themselves. As such, the files present on the ftp server should be regarded as the definitive inventory of the data set. 12) REFERENCES -------------------------------------------------------------Paget MJ and King EA (2008). MODIS Land data sets for the Australian region. CSIRO Marine and Atmospheric Research Internal Report 004. Land Processes Distributed Active Archive Center (LPDAAC). http://lpdaac.usgs.gov 13) CONTACTS -------------------------------------------------------------June 2008 Mr Matt Paget, CSIRO Marine and Atmospheric Research matt.paget@csiro.au Dr Edward King, CSIRO Marine and Atmospheric Research edward.king@csiro.au 97 Appendix 3 Summary of literature review of in-stream biota ecology relevant to water scarcity Notes on methods and scope This section reports on information from the scientific literature. The literature search was limited to post-1999 papers published in English only, using multiple databases accessed via “CrossSearcher” (Expanded Academic ASAP (Gale); InfoTrac Onefile (Gale), Science Direct (Elsevier), Wiley Interscience (inc. Blackwell), Web of Science, Ecology Abstracts (CSA). Various combinations of the following terms were used: water stress, ecological indicator, water scarcity, drought, ecological impacts, macroinvertebrates, fish, flow, review, freshwater ecology, stream biota, river biota. This review focuses on research reporting relationships between hydrology/flow variables (from mostly for flowing waters (i.e. rivers), but relevant research on wetlands or lakes also included), particularly with respect to reduced or altered flows; and for the most commonly monitored freshwater biota, namely macroinvertebrates and fish. However, selected research publications on other biota found incidentally during the literature search that was deemed to be relevant has also been included (e.g. zooplankton, diatoms, other vertebrates, other algae). The initial search resulted in a shortlist of approximately 250 papers. Abstracts were read in order to refine the list further; the remaining 94 papers were used to prepare this review. Very few published papers were found to report on the use of a particular faunal group specifically as indicators of water scarcity, but a substantial body of literature exists on the topic of using freshwater biota for more general environmental monitoring (and especially detection of pollution). Biology and ecology of aquatic biota relevant to water scarcity The flow regime is widely regarded to be the key driver of river and floodplain wetland ecosystems (Bunn and Arthington 2002). Variable flows are a natural feature of many Australian river systems and aquatic biota have evolved 98 under these conditions. Furthermore, drought forms an integral part of the Australian climate, and has played an important role in promoting diversity within its unique aquatic ecosystems (Rose et al. 2008; Sheldon and Thoms 2006). However, there is considerable evidence that human activities across catchments and in water bodies (i.e. land clearing, grazing, urbanisation, river regulation, water extraction) have served to exacerbate the impacts of, and delay the ecological recovery from drought (Bond et al. 2008). Australian stream biota have adapted to become resistant (able to survive dry periods) or resilient (have efficient recovery mechanisms) to periods of water scarcity (Boulton 2003). For example, some macroinvertebrates have drought resistant traits, such as resistant forms (e.g. cocoons, desiccation resistant egg masses), spiracular respiration, very large size, aerial dispersal and short life cycles (Bonada et al. 2007). Once surface flow disappears (as a result of on-going drought or anthropogenic activity e.g. water abstraction), streams are often reduced to a series of isolated pools and refugia become critical for the survival of aquatic organisms (Boulton 2003). Important refugia include the hyporheic zone and spring-fed pools (for macroinvertebrates and fish), seeps, leaf litter, dry sediment and exposed riffle zones (dry biofilm) (for algae and plankton) (Boulton 2003; Magoulick and Kobza 2003; Robson and Matthews 2004). Mechanisms underlying the impact of water scarcity on in-stream biota Reduced flow or discharge can lead to changes in physical, chemical and biological characteristics of stream environments. These changes often equate to reduced habitat quality or availability for in-stream biota. A conceptual model for the Cotter River, ACT (Cottingham et al. 2005) illustrates the mechanisms by which reduced flows may impact on macroinvertebrates and fish (Figure A3.1). 99 Figure A3.1. Ecological responses expected when flow is reduced in the Cotter River, ACT (from Cottingham et al. 2005). Reduced flows result in riparian vegetation encroaching into the channel, thereby reducing channel capacity (1). Low flows and a reduced frequency of flushing are considered to have increased the retention of nutrients and fine sediment, resulting in conditions favourable for the growth of filamentous algae and biofilms that are unpalatable for macroinvertebrates (2). Reduced flow also results in armouring, reduced flushing of detritus, nutrients, fine sediment (3). These changes, along with areas of the streambed becoming exposed, lead to a reduction in habitat availability and quality for macroinvertebrates and fish in the substratum (4). Habitat quality may also be reduced (through changes to water quality) because sediment and organic matter entering the channel directly from adjacent valley slopes may not be flushed by low flows in the main channel (Cottingham et al. 2005). 100 In the case of algae and plankton, a conceptual model presented by Burns and Ryder (2001), which illustrated how changes to flow characteristics (as a form of physical disturbance) affect biofilms, is also useful for understanding the mechanism of impact for water scarcity on group of organisms (Figure A3.2). Flow and water level act as resource modulators for biofilms through their influence on nutrient and light availability, and by clearing substrata through scouring and abrasion. Hence, flow regulates accrual of biofilm biomass in river systems. Currents directly affect biofilms through scour and substratum loss (which indirectly break boundary layers that impede nutrient uptake) and by altering light attenuation through differences in loads of suspended solids. Based on this conceptual model, it can be inferred that the main mechanisms by which water scarcity can affect biofilm production is through an increase in light levels and reduced exposure to substrates. Based on this conceptual model, it can be inferred that one of the main mechanisms by which water scarcity can affect biofilm production is through an increase in light levels. 101 Figure A3.2. Components of community structure and function important in determining biomass, composition and physiology of riverine biofilms. Matter and energy flow are connected by solid arrows that indicate ‘processes’. The ‘modulators’ of community function are indicated by arrows originating from small circles (---). Physical disturbances are derived from the community modulators such as flow (top) and grazers (below). Essential resources for biofilm growth are shown in the second layer of the diagram (Burns and Ryder 2001). 102 Relationships between hydrology and ecology A number of studies have investigated relationships between hydrology and ecology, for in-stream biota. The studies most relevant to the issue of water scarcity are those that assessed impacts of (a) reduced flows, discharge or drought, or (b) flow alteration, on in-stream biota. Recent reviews on the ecological impacts of reduced flows or drought include (Lake 2003), (Matthews and Marsh-Matthews 2003), (Dewson et al. 2007), (Bond et al. 2008), (Rose et al. 2008). Reviews on the more general issue of flow alteration include (Petts 1984), (Walker 1985), (Kingsford 2000), (Bunn and Arthington 2002), (Lloyd et al. 2003), (Biggs et al. 2005), (Welcomme et al. 2006a), and (Poff et al. 2009). Several examples of quantitative research linking hydrological and ecological data were also identified: Macroinvertebrates – (DeGasperi et al. 2009; Gibbins et al. 2001; Jowett and Duncan 1990; Konrad et al. 2008; Lorenz et al. 2004; Monk et al. 2006; Rempel et al. 2000; Shivoga 2001) Fish – (Growns 2008; Kennard et al. 2007; King et al. 2009; Knight et al. 2008; Mannes et al. 2008; Roy et al. 2005) Algae/Plankton – (Dickerson et al. 2009; Maltchik and Medeiros 2006; Matveev and Matveeva 2005; Thorp and Mantovani 2005) The LIFE approach (Lotic-invertebrate Index for Flow Evaluation; (Extence et al. 1999), for assessing the impact of variable flows on benthic macroinvertebrate populations, is highly relevant and warrants a more detailed discussion. The LIFE method was developed using data from a number of English rivers and is primarily based on the known flow preferences of selected British benthic macroinvertebrates. Commonly identified British freshwater species were allocated into one of six flow groups (see Table A3.1). 103 Table A3.1. Benthic freshwater macroinvertebrate flow groups, ecological associations and defined current velocities (Extence et al. 1999). Group Ecological flow association Mean current velocity I Taxa primarily associated with rapid flows Typically > 100 cm s-1 II Taxa primarily associated with moderate to fast flows Typically 20–100 cm s-1 III Taxa primarily associated with slow or sluggish flows Typically < 20 cm s-1 IV Taxa primarily associated with flowing (usually slow) and standing waters — V Taxa primarily associated with standing waters — VI frequently associated with drying or drought impacted sites — Statistical modelling was used to explore relationships between several hundred flow variables and LIFE scores. This process identified a subset of flow variables that were of critical importance in influencing community structure in different rivers. Combinations of the following flow measures were examined for comparison with long-term LIFE values: 1. Flow statistics (e.g. percentile flow, mean flow, maximum flow, minimum flow, etc. Over various time scales); 2. Flow duration (e.g. 90, 120, 150 days, etc); 3. Flow period (e.g. full year, April–September, March–October, etc) (Extence et al. 1999). The architects of the LIFE approach advocate that their technique is suitable for assessing the effects of low flows, as well as abstraction and augmentation outputs. In addition, they suggest the LIFE method could provide a basis for setting benchmark flows suitable for protecting and maintaining ecological integrity (Extence et al. 1999). The LIFE approach has not been adopted by monitoring agencies in the UK, however, some further research investigating the application of this technique has been published (e.g. (Clarke et al. 2003; Greenwood et al. 2006; Monk et al. 2006). 104 Responses of in-stream biota to water scarcity The effects of reduced flow or drought on in-stream biota are presented in detail earlier in the report (Table 4.1- 4.4). To recap, the most frequently reported responses of animals (i.e. macroinvertebrates, fish, other vertebrates or invertebrates) to water scarcity were (i) decreases in diversity and abundance, (ii) changes to community composition, (iii) reduced reproductive success. In particular, riffle habitat specialists tended to be replaced by poolpreferring taxa, as flow or discharge decreased. For algae or plankton, reduced flow or drought was associated with increases in algal biomass, decreases in diversity and altered community structure. Groundwater dependence Some species of algae and macroinvertebrates may be useful indicators of groundwater dependency for base flows in rivers (Boulton and Hancock 2006). The river–groundwater connectivity can also create an important habitat mosaic that sustains biodiversity in floodplains (Brunke et al. 2003). Riparian bird diversity Regardless of the habitat type, we know that in Australia, all bird richness is related to water availability (Hawkins et al. 2005) and there are fewer species in drier areas (Hawkins et al. 2005). The potential of using all birds as indicators is highlighted by (Jansen and Robertson 2001) who found that riparian zones support higher abundance and diversities of birds than nonriparian habitats. There is a clear link between riparian bird communities and in stream degradation (Hughes et al. 2009) and riparian birds were added to the list of routine indicators for stream ecosystem bioassessment in Oregon, USA (Bryce et al. 2002). Yet these links and indicators are always more generally than just water related stress. The indicator developed by Bryce et al. (2002) is for generic assessment not just hydrological condition; the link made by Hughes et al. (2009) results from global and local processes, and the results from Jansen ad Robertson (2001) are somewhat muddied by grazing and habitat clearance making them less sensitive to water balance alone. 105 Acknowledgements Substantial contributions by literature collation and summaries were made by Kerry Beggs and Nicole McCasker. Expert advice was given by many, but particular guidance was given by Edward King, Jane Roberts and Neil Sims. 106 References Angradi T. R., Bolgrien D. W., Jicha T. M., Pearson M. S., Hill B. H., Taylor D. L., Schweiger E. W., Shepard L., Batterman A. R., Moffett M. F., Elonen C. M. & Anderson L. E. (2009) A bioassessment approach for mid-continent great rivers: the Upper Mississippi, Missouri, and Ohio (USA). Environmental Monitoring and Assessment, 152: 425–42. Attrill M. J., Rundle S. D. & Thomas R. M. (1996) The influence of droughtinduced low freshwater flow on an upper-estuarine macroinvertebrate community. Water Research, 30: 261-8. Bader M. (2000) Productivity-biodiversity patterns – a study using multitemporal Landsat TM NDVI data for the Alice Springs region, central Australia., Centre for Geo-Information, WUR, Wageningen, Thesis Report GIRS-2000-28-MB. Barbour M. T., Plafkin J. L., Bradley B. P., Graves C. G. & Wisseman R. W. (1992) Evaluation of EPA's rapid bioassessment benthic metrics: metric redundancy and variability among reference stream sites. Environmental Toxicology and Chemistry, 11: 437-49. Bellio M. G., Kingsford R. T. & Kotagama S. W. (2009) Natural versus artificial- wetlands and their waterbirds in Sri Lanka. Biological Conservation, 42: 3076–85. Biggs B. J. F., Nikora V. I. & Snelder T. H. (2005) Linkning scales of flow variability to lotic ecosystem structure and function. River Research and Applications, 21: 283–98. Blinn D. W. & Bailey P. C. E. (2001) Land-use influence on stream water quality and diatom communities in Victoria, Australia: a response to secondary salinization. Hydrobiologia, 466: 231–44. 107 Bonada N., Rieradevall M. & Prat N. (2007) Macroinvertebrate community structure and biological traits related to flow permanence in a Mediterranean river network. Hydrobiogia, 589: 91-106. Bond N. R., Lake P. S. & Arthington A. H. (2008) The impacts of drought on freshwater ecosystems: an Australian perspective. Hydrobiologia, 600: 3–16. Boulton A. J. (2003) Parallels and contrasts in the effects of drought on macroinvertebrate assemblages. Freshwater Biology, 48: 1173–85. Boulton A. J. & Hancock P. J. (2006) Rivers as groundwater-dependent ecosystems: a review of degrees of dependency, riverine processes and management implications. Australian Journal of Botany, 54: 133–44. Boulton A. J. & Lloyd L. N. (1992) Flooding frequency and invertebrate emergence from dry floodplain sediments of the River Murray, Australia. Regulated Rivers, 7: 137-51. Bradford M. J. (1997) An experimental study of stranding of juvenile salmonids on gravel bars and in side channels during rapid flow fluctuations. Regulated Rivers: Research and Management, 13: 395-401. Bradford M. J. & Heinonen J. S. (2008) Low flows, instream flow needs and fish ecology in small streams. Canadian Water Resources Journal, 33: 16580. Bradford M. J., Taylor G. C., Allan J. A. & Higgins P. S. (1995) An experimental study of stranding of juvenile coho salmon and rainbow trout during rapid flow decreases in winter conditions. North American Journal of Fisheries Management, 15: 473-9. Briggs S. V., Thornton S. A. & Lawler W. G. (1997) Relationships Between Hydrological Control of River Red Gum Wetlands and Waterbird Breeding. Emu, 97: 31-42. Brunke M., Hoehn E. & Gonser T. (2003) Patchiness of River–Groundwater Interactions within Two Floodplain Landscapes and Diversity of Aquatic Invertebrate Communities. Ecosystems, 6: 707–22. 108 Bunn S. & Davies P. M. (2000) Biological processes in running waters and their implications for the assessment of ecological integrity. hydrobiogia, 422/423: 61-70. Bunn S., Davies P. M. & Mosisch T. D. (1999) Ecosystem masures of river health and their response to riparian and catchment degradation. Freshwater Biology, 41: 333-45. Bunn S. E. & Arthington A. H. (2002) Basic Principles and Ecological Consequences of Altered Flow Regimes for Aquatic Biodiversity. Environmental Management, 30: 492–507. Burns A. & Ryder D. (2001) Potential for biofilms as biological indicators in Australian riverine systems. Ecological management and restoration, 2: 53-63. Cadwallader P. L. & Lawrence B. ( 1990) Fish. In: The Murray (eds N. Mackay and D. Eastburn) pp. 316- 35. Murray-Darling Basin Commission, Canberra. Capon J., James C. S., Williams L. & Quinn G. P. (2009) Responses to flooding and drying in seedlings of a common Australian desert floodplain shrub: Muehlenbeckia florulenta Meisn. (tangled lignum) Environmental and Experimental Botany, 66. Cash W. B. & Holberton R. L. (2005) Endocrine and Behavioral Response to a Decline in Habitat Quality: Effects of Pond Drying on the Slider Turtle, Trachemys scripta. Journal of Experimental Zoology, 303A: 872–9. Chambers L. E. (2008) Trends in timing of migration of south-western Australian birds and their relationship to climate. Emu, 109: 1-14. Chessman B. (2009) Climatic changes and 13-year trends in stream macroinvertebrate assemblages in New South Wales, Australia. Global Change Biology, 15: 2791-802. Chessman B. C. (1995) Rapid assessment of rivers using macroinvertebrates: a procedure based on habitat specific sampling, family level identification and a biotic index. Australian Journal of Ecology, 20: 122-9. 109 Clarke R. T., Armitage P. D., Hornby D., Scarlett P. M. & Davy-Bowker J. (2003) Investigation of the Relationship between the LIFE Index and RIVPACS: Putting LIFE into RIVPACS. Technical Report. . Environment Agency. (Abstract only - From Bournemouth university website http://eprints.bournemouth.ac.uk/7798/). Cottingham P., Quinn G., Norris R., King A., Chessman B. & Marshall C. (2005) Environmental flows monitoring and assessment framework. Technical Report. CRC for Freshwater Ecology, Canberra. Covich A. P., Crowl T. A. & Scatena F. N. (2003) Effects of extreme low flows on freshwater shrimps in a perennial tropical stream. Freshwater Biology, 48: 1199–206. Cowx I. G., Young W. O. & Hellawell J. M. (1984) The influence of drought on the fish an invertebrate populations of an upland stream in Wales. Freshwater Biology, 14: 165–77. Crome F. H. J. (1988) To Drain or Not to Drain? - Intermittent Swamp Drainage and Waterbird Breeding. Emu, 88: 243-8. Cunningham S. C., Mac Nally R., White M., Read J., Baker P. J., Thomson J. & Griffioen P. (2007a) Mapping the current condition of River Red Gum (Eucalyptus camaldulensis Dehnh.) stands along the Victorian Murray River Floodplain. A Report to the Northern Victorian Catchment Management Authorities and the Department of Sustainability and Environment. . Cunningham S. C., Read J., Baker P. J. & MacNally R. (2007b) Quantitative assessment of stand condition and its relationship to physiological stress in stands of Eucalyptus camaldulensis (Myrtaceae). Australian Journal of Botany, 55: 692-9. Cunningham S. C., Thomson J. R., Read J., Baker P. J. & MacNally R. (2009) Does stand structure influence susceptibility of eucalypt floodplain forests to dieback? Austral Ecology, early edition on line. 110 Davies P. E. (2000) Assessing the biological quality of freshwaters. In: Development of a National River Bioassessment System (AUSRIVAS) in Australia (eds J. R. Wright, D. W. Sutcliffe and M. T. Furse) pp. 113-24. Freshwater Biological Association, Cumbria. DeGasperi C. L., Berge H. B., Whiting K. R., Burkey J. J., Cassin J. L. & Fuerstenberg R. R. (2009) Linking hydrologic alteration to biological impairment in urbanizing streams of the Puget Lowland, Washington, USA. JOURNAL OF THE AMERICAN WATER RESOURCES ASSOCIATION, 45: 512-33. DOI: 10.1111/j.752-688.2009.00306.x. Department of the Environment Water Heritage and the Arts. (2008) National Framework and Guidance for Describing the Ecological Character of Australia’s Ramsar Wetlands. Module 2 of the National Guidelines for Ramsar Wetlands—Implementing the Ramsar Convention in Australia. Australian Government, Department of the Environment, Water, Heritage and the Arts, Canberra. Desgranges J.-L., Ingram J., Drolet B., Morin J., Savage C. & Borcard D. (2006) Modelling wetland bird repsonse to water level changes in the Lake Ontario - St Lawrenece River hydrosystem. Environmental Monitoring and Assessment, 113: 329-65. Dewson Z. S., James A. B. W. & Death R. G. (2007) A review of the consequences of decreased flow for instream habitat and macroinvertebrates. Journal of the North American Benthological Society, 26: 401-15. Dickerson K. D., Medley K. A. & Havel J. E. (2009) Spatial varation in zooplankton community structure is related to hydrologic flow units in the Missouri River, USA. River Research and Applications: DOI: 10.1002/rra.268. Dorfman E. J., Lamont A. & Dickman C. R. (2001) Foraging behaviour and success of Black-necked Storks (Ephippiorhynchus asiaticus) in Australia: implications for management. Emu, 101: 145-9. Edgar B. (2008) Stocktake of river health monitoring programs. 111 Eglington S. M., Gill J. A., Bolton M., Smart M. A., Sutherland W. J. & Watkinson A. R. (2008) Restoration of wet features for breeding waders on lowland grassland. Journal of Applied Ecology, 45: 305-14. Extence C. A., Balbi D. M. & Chadd R. P. (1999) River flow indexing using British benthic macroinvertebrates: a framework for setting hydroecological objectives. Regulated Rivers: Research and Management, 15: 543–74. Fellows C. S., Clapcott J. E., Udy J. W., Bunn S. E., Harch B. D. & Davies P. M. (2006) Benthic metabolism as an indicator of stream ecosystem health. Hydrobiogia, 572: 71–87. Fjeldsa J. (1985) Classification of waterbird communities in South-eastern Austrlaia. Emu, 85: 141-9. Follner K., Hofacker A., Glaeser J., Dziock F., Gerisch M., Foeckler F., Ilg C., Schanowski A., Sscholz M. & Henle K. (2009) Accurate environmental bioinication in floodplains in spite of an extreme flood event. River Research and Applications: DOI: 10.1002/rra.300. Freeman M. C., Bowen Z. H., Bovee K. D. & Irwin E. R. (2001) Flow and Habitat Effects on Juvenile Fish Abundance in Natural and Altered Flow Regimes. Ecological Applications, 11: 179-90. Freeman M. C. & Marcinek P. A. (2006) Fish Assemblage Responses to Water Withdrawals and Water Supply Reservoirs in Piedmont Streams. Environmental Management, 38: 435–50. Geddes M. C. & Puckridge J. T. (1989) Survival and growth of larval and juvenile native fish: The importance of the floodplain. In: Proceedings of a workshop on native fish management, Canberra pp. 101-16. Murray-Darling Basin Commission, Canberra. Georges A., Webster E., Guarino E., Thoms M., Jolley P. & Doody J. S. (2003) Modeling dry season flows and predicting the impact of water extraction on a flagship species. Final Report to DLPE NT. Applied Ecology Research Group and CRC for Freshwater Ecology, University of Canberra. 112 Gessner M. O. & Chauvet E. (2002) A case for using litter breakdown to assess functional stream integrity. Ecological Applications, 12: 498–510. Gibbins C. N., Dilks C. F., Malcolm R., Soulsby C. & Juggins S. (2001) Invertebrate communities and hydrologic variation in Cairngorm mountain streams. Hydrobiologia, 462: 205-19. Gippel C. (2007) Stocktake of river health protocols. Golladay S. W., Gagnon P., Kearns M., Battle J. M. & Hicks D. W. (2004) Response of freshwater mussel assemblages (Bivalvia: Unionidae) to a record drought in the Gulf Coastal Plain of southwestern Georgia. Journal of the North American Benthological Society, 23: 494-506. Green A. J., El Hamzaoui M., El Agbani M. A. & Franchimont J. (2002) The conservation status of Moroccan wetlands with particular reference to waterbirds and to changes since 1978. Biological Conservation, 104: 71-82. Greenwood M. T., Wood P. J. & Monk W. A. (2006) The use of fossil caddisfly assemblages in the reconstruction of flow environments from floodplain paleochannels of the River Trent, England. Journal of Paleolimnology, 35: 747-61. Growns I. (2008) The influence of changes to river hydrology on freshwater fish in regulated rivers of the Murray–Darling basin. Hydrobiologia, 596: 203– 11. Halse S. A. & Jaensch J. P. (1989) Breeding Seasons of Waterbirds in Southwestern Australia - the Importance of Rainfall. Emu, 89: 232-49. Harris J. H. (1988) Demography of Australian bass, Macquaria novemaculeata (Perciformes, Percichthyidae) in the Sydney basin. Australian Journal of Marine and Freshwater Research, 39: 355-69. Harris J. H. & Gehrke P. (1996) Fish IBI. Hering D., Moog O., Sandin L. & Verdonschot P. F. M. (2004) Overview and application of the AQEM assessment system. Hydrobiologia, 516: 1–20. 113 Hill M. J., Senarath U., Lee A., Zeppel M., Nightingale J. M., Williams R. D. J. & McVicar T. R. (2006) Assessment of the MODIS LAI product for Australian ecosystems. Remote Sensing of Environment, 101: 495-518. Holland K. L., Charles A. H., Jolly D., Overton I. C., Gehrig S. & Simmons C. T. (2009) Effectiveness of artificial watering of a semi-arid saline wetland for managing riparian vegetation health. Hydrological Processes, 23: 3474-84. Horner G. J., Baker P. J., MacNally R., Cunningham S. C., Thomson J. R. & Hamilton F. (2009) Mortality of developing floodplain forests subjected to a drying climate and water extraction. Global Change Ecology, 15. Hose G. C., Jones P. & Lim R. P. (2005) Hyporheic macroinvertebrates in riffle and pool areas of temporary streams in south eastern Australia. Hydrobiologia, 532: 81-90. Hughes L. (2003) Climate change and Australia: Trends, projections and impacts. Austral Ecology, 28: 423-43. Hughes S. J., Santos J. M., Ferreira M. T., R C. & Mendes A. M. (2009) Ecological assessment of an intermittent Mediterranean river using community structure and function: evaluating the role of different organism groups. Freshwater Biology, 54: 2383–400. James A. B. W., Dewson Z. S. & Death R. G. (2009) The influence of flow reduction on macroinvertebrate drift density and distance in three New Zealand streams. Journal of the North American Benthological Society , 28: 220-32. Jensen A. E., Walker K. F. & Paton D. C. (2006) The role of seedbanks in restoration of floodplain woodlands. River Research and Applications, 24: 632-49. Jensen A. E., Walker K. F. & Paton D. C. (2008) The role of seedbanks in restoration of floodplain woodlands. River Research and Applications, 24: 632-49. 114 Johnson R. K., Hering D., Furse M. T. & Verdonschot P. F. M. (2006) Indicators of ecological change: comparison of the early response of four organism groups to stress gradients. Hydrobiologia, 566: 39–152. Jolly I. D., McEwan K. L. & Holland K. L. (2008) A review of groundwater– surface water interactions in arid/semi-arid wetlands and the consequences of salinity for wetland ecology. Ecohydrology, 1. Jowett I. G. & Duncan M. J. (1990) Flow variability in New Zealand rivers and its relationship to in-stream habitat and biota. New Zealand Journal of Marine and Freshwater Research, 24: 305-17. Jubb R. A. (1972) The J.G. Strydom Dam: Pongolo River: northern Zululand. The importance of floodplain pans below it. Piscator, 86: 104-9 (cited in Bunn and Arthington 2002). Katz G. L., Stromberg J. C. & Denslow M. W. (2009) Streamside herbaceous vegetation response to hydrologic restoration on the San Pedro River, Arizona. Ecohydrology, 2: 213-25. Kennard M. J., Olden J. D., Arthington A. H., Pusey B. J. & Poff N. L. (2007) Multiscale effects of flow regime and habitat and their interaction on fish assemblage structure in eastern Australia. Canadian Journal of Fisheries and Aquatic Sciences, 64: 1346–59. doi:10.139/F07-108. King A. J., Tonkin Z. & Mahoney J. (2009) Environmental flow enhances native fish spawing and recruitment in the Murray River, Australia. River Research and Applications, 25: 1205–18. DOI: 10.002/rra.9. King E. A. (2003) The Australian AVHRR Data Set at CSIRO/EOC: Origins, Processes, Holdings and Prospects. CSIRO Earth Observation Centre Report 2003/04. Kingsford R. T. (2000) Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecology, 25: 109–27. 115 Kingsford R. T. & Auld K. M. (2005) Waterbird Breeding and Environemntal Flow Management in the Macquarie Marshes, Arid Australia. River Research and Applications, 21: 187-200. Kingsford R. T., Curtin A. L. & Porter J. (1999) Water flows on Cooper Creek in arid Australia determine `boom' and `bust' periods for waterbirds. Biological Conservation, 88: 231-48. Kingsford R. T. & Johnson W. (1998) Impact of Water Diversions on ColoniallyNesting Waterbirds in the Macquarie Marshes of Arid Australia Colonial Waterbirds, 21: 159-70 Kingsford R. T. & Lee E. (2007) Review of floodplain health with regard to bird breeding and habitat provision for the Murray Darling Basin across icon site, valley zone and valley scales. University of New South Wales, UNSW Biol. 2007_Jun_1, Sydney. Kingsford R. T. & Norman F. I. (2002) Australian waterbirds – products of the continent’s ecology. Emu, 102: 47-69. Kingsford R. T. & Thomas R. F. (2004) Destruction of Wetlands and Waterbird Populations by Dams and Irrigation on the Murrumbidgee River in Arid Australia. Environmental Management, 34: 383-96. Knight R. R., Gregory M. B. & Wales A. K. (2008) Relating streamflow characteristics to specialized insectivores in the Tennessee River Valley: a regional approach. Ecohydrology, 1: 394– 407. DOI: 10.1002/eco.32. Konrad C. P., Brasher A. M. D. & May J. T. (2008) Assessing streamflow characteristics as limiting factors on benthic invertebrate assemblages in streams across the western United States. Freshwater Biology, 53: 1983–98. Kushlan G. A. (1976) Environmental stability and fish community diversity. Ecology, 57: 821-5. 116 Kushlan K. A. (1993) Colonial Waterbirds as Bioindicators of Environmental Change. Colonial Waterbirds, 16: 223-51 Lake P. S. (1975) Fish of the Murray River. In: The book of the Murray (eds G. C. Lawrence and G. K. Smith) pp. 213-24. Rigby, Adelaide. Lake P. S. (2003) Ecological effects of perturbation by drought in flowing waters. Freshwater Biology, 48: 1161–72. Lasne E., Bergerot B., Lek S. & Laffaille P. (2007) Fish zonation and indicator species for the evaluation of the ecological status of rivers: example of the Loire Basin (France). River Research and Applications, 23: 877–90. Lesley D. J. (2001) Effect or River Management on Colonially-nesting Waterbirds in the Barmah-Millewa Forest, South-eastern Australia. Regulated Rivers: Research and Management, 17: 21-36. Lind P. R., Robson B. J. & Mitchell B. D. (2006) The influence of reduced flow during a drought on patterns of variation in macroinvertebrate assemblages across a spatial hierarchy in two lowland rivers. Freshwater Biology, 51: 2282– 95. Lloyd N., Quinn G., Thoms M., Arthington A., Gawne B., Humphries P. & Walker K. (2003) Does flow modification cause geomorphological and ecological response in rivers? A literature review from an Australian perspective. CRC for Freshwater Ecology, Canberra. Lobo A., IBAÂ NÄ EZ MARTIÂ J. J. & CARRERA GIMEÂ NEZ-CASSINA C. (1997) Regional scale hierarchical classiffcation of temporal series of AVHRR vegetation index. International journal of Remote Sensing, 18: 3167-93. Lorenz A., Hering D., Feld C. K. & Rolauffs P. (2004) A new method for assessing the impact of hydromorphological degradation on the macroinvertebrate fauna of five German stream types. Hydrobiologia, 516: 107–27. Lowe-McConnell R. H. (1985) Ecological studies in tropical fish communities. Cambridge University Press, Cambridge. 117 LPDAAC. (2010) Land Processes Distributed Active Archive Center, http://lpdaac.usgs.gov/citation.asp, Accessed 14th March. In: . Magoulick D. D. & Kobza R. (2003) The role of refugia for fishes during drought: a review and synthesis. Freshwater Biology, 48: 1186-98. Malmqvist B. & Sackmann G. (1996) Changing risk of predation for a filterfeeding insect along a current velocity gradient. Oecologia (Berlin), 108: 4508. Maltchik L. & Medeiros E. S. F. (2006) Conservation importance of semi-arid streams in north-eastern Brazil: implications of hydrological disturbance and species diversity. Aquatic Conservation: Marine and Freshwater Ecosystems, 16: 665–77. Mannes S., Robinson C.-T., Uehlinger U., Scheurer T., Ortlepp J., Mürle U. & Molinari P. (2008) Ecological effects of a long-term flood program in a flowregulated river. Revue de géographie alpine (Journal of Alpine Research) [Online], 96-1: 125-34. URL : http://rga.revues.org/index450.html. Marchant R. & Hehir G. (2002) The use of AUSRIVAS predictive models to assess the response of lotic macroinvertebrates to dams in south-east Australia. Freshwater Biology, 47: 1033–50. Marchetti M. P. & Moyle P. B. (2001) Effects of Flow Regime on Fish Assemblages in a Regulated California Stream. Ecological Applications, 11: 530-9. Matczak T. D. & Mackay R. J. (1990) Territoriality in filter-feeding caddisfly larvae: laboratory experiments. Journal of the North American Benthological Society, 9: 26-34. Mathews. (2009). Matthews W. J. & Marsh-Matthews E. (2003) Effects of drought on fish across axes of space, time and ecological complexity. Freshwater Biology, 48: 1232– 53. 118 Mattsson B. J. & Cooper R. J. (2006) Louisiana waterthrushes (Seiurus motacilla) and habitat assessments as cost-effective indicators of instream biotic integrity. Freshwater Biology, 51: 1941-58. Matveev V. F. & Matveeva L. K. (2005) Seasonal succession and long-term stability of a pelagic community in a productive reservoir. Marine and Freshwater Research, 56: 1137–49. McDonald M. E., Paulsen S., Blair R., Dlugosz J., Hale S., Hedtke S., Heggem D., Jackson L., Jones K. B., Levinson B., Olsen A., Stoddard J., Summers K. & Veith G. (2002) Research strategy: Environmental Monitoring and Assessment Program. US EPA, Office of Research and Development, Research Triangle Park, NC. McVicar T. R., Briggs P. R., A. K. E. & Raupach M. R. (2003) A review of predictive modelling from a natural resource management perspective: The role of remote sensing of the terrestrial environment. A report to the Bureau of Rural Sciences by CSIRO Land and Water and the CSIRO Earth Observation Centre, September. MDBC. (2008) The Living Muuray: Icon Site Condition Report October 2008. Murray-Darling Basin Commission, Canberra. Medeiros E. S. F. & Maltchik L. (1999) The effects of hydrological disturbance on the intensity of infestation of Lernaea cyprinacea in an intermittent stream fish community. Journal of Arid Environments, 43: 351-6. Merritt D. M., Scott M. L., Poff N. L., Auble G. T. & Lytle D. A. (2010) Theory, methods and tools for determining environmental flows for riparian vegetation: riparian vegetation-flow response guilds. Freshwater Biology, 55: 206–25. Monk W. A., Wood P. J., Hannah D. M., Wilson D. A., Extence C. A. & Chadd R. P. (2006) Flow variability and macroinvertebrate community response within riverine systems. River Research and Applications, 22: 595–615. Nielsen D., Watson G. & Petrie R. (2005) Microfaunal communities in three lowland rivers under differing flow regimes. Hydrobiologia, 543: 101–11. 119 Nilsson C. & Dynesius M. (1994) Ecological effects of river regulation on mammals and birds: A review. Regulated Rivers: Research and Management, 9: 45-54. Noordhuis R., van der Molen D. T. & van den Berg M. S. (2002) Response of herbivorous water-birds to the return of Chara in Lake Veluwemeer, The Netherlands Aquatic Botany, 72: 349-67. Norris R. H., Dyer F., Hairsine P., Kennard M., Linke S., Merrin L., Read A., Robinson W., Ryan C., Wilkinson S. & Williams D. (2007a) A baseline assessment of water resources for the National Water Initiative, Level 2 Assessment. Assessment of River and Wetland Health: A Framework for Comparative Assessment of the Ecological Condition of Australian Rivers and Wetlands. . National Water Commission, 44pp. Norris R. H., Dyer F., Hairsine P., Kennard M., Linke S., Merrin L., Read A., Robinson W., Ryan C., Wilkinson S. & Williams D. (2007b) A baseline assessment of water resources for the National Water Initiative, Level 2 Assessment. Assessment of River and Wetland Health: Potential Comparative Indices. National Water Commission, 143 pp. Norris R. H., Prosser I., Young B., Liston P., Bauer N., Davies N., Dyer F., Linke S. & Thoms M. (2001) The Assessment of River Condition (ARC): An audit of the ecological condition of Australian rivers. Final Report submitted to the National Land and Water Resources Audit Office. p. 289. Cooperative Research Centre for Freshwater Ecology and Commonwealth Scientific and Industrial Research Organization, Division of Land and Water, Canberra. Oberdorff T., Pont D., Hugueny B. & Porcher J.-P. (2002) Development and validation of a fish-based index (FBI) for the assessment of ''river health'' in France. Freshwater Biology, 47: 1720-34. Osmundson D. B., Ryel R. J., Lamarra V. L. & Pitlick J. (2002) Flow-sedimentbiota relations: implications for river regulation effects on native fish abundance. Ecological Applications, 12: 1719–39. 120 Osmunson D. B., Ryel R. J., Lamarra V. L. & Pitlick J. (2002) Flow-sedimentbiota relations: implications for river regulation effects on native fish abundance. Ecological Applications, 12: 1719–39. Overton I. C., Collof M., Doody T. M., Henderson B. & Cuddy S. M. (2009) Ecological Outcomes of Flow Regimes in the Murray Darling Basin. Report prepared for the National Water commission by CSIRO Water for a healthy Country Flagship. Overton I. C., Jolly I. D., Slavich P. G., Lewis M. M. & Walker G. R. (2006) Modelling vegetation health from the interaction of saline groundwater and flooding on the Chowilla floodplain, South Australia. Australian Journal of Botany, 54: 207-20. Paget M. J. & King E. A. (2008) MODIS Land data sets for the Australian region. CSIRO Marine and Atmpospheric Research Internal Report No. 004. Paillisson J.-M., Reeber S. & Marion L. (2002) Bird assemblages as bioindicators of water regime management and hunting disturbance in natural wet grasslands. Biological Conservation, 106: 115-27. Petts G. E. (1984) Impounded rivers. Perspectives for ecological management. John Wiley & Sons, Chichester, UK (cited in Bunn and Arthington 2002). Poff N. L., Richter B. D., Arthington A. H., Bunn S. E., Naiman R. J., Kendy E., Acreman M., Apse C., Bledsoe B. P., Freeman M. C., Henriksen J., Jacobson R. B., Kennen J. G., Merritt D. M., O’Keeffe J. H., Olden J. D., Rogers K., Tharme R. E. & Warner A. (2009) The ecological limits of hydrologic alteration (ELOHA): a new framework for developing regional environmental flow standards. Freshwater Biology: doi:10.1111/j.365-2427.009.02204.x. Poff N. L. & Zimmerman J. (2010) Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshwater Biology, 55: 194-205. 121 Pringle C. M. & Scatena F. N. (1999) Freshwater resource development. Case studies from Puerto Rico and Costa Rica. In: Managed ecosystems: The Mesoamerican experience (eds L. U. Hatch and M. E. Swisher) pp. 114-21. Oxford University Press, New York (cited in Bunn and Arthington 2002). Pusey B., Kennard M. & Arthington A. (2004) Freshwater Fishes of North- eastern Australia. CSIRO Publishing, Collingwood, VIC. Reid M. A. & Brooks J. J. (2000) Detecting effects of environmental water allocations in wetlands of the Murray-Darling Basin, Australia. Regulated Rivers: Research and Management, 16: 479–96. Reitan O. & Sandvik J. (1996) An assessment of retaining dams in hydropower reservoirs for enhancing bird habitat. Regulated Rivers: Research and Management, 12: 523-34. Rempel L. L., Richardson J. S. & Healey M. C. (2000) Macroinvertebrate community structure along gradients of hydraulic and sedimentary conditions in a large gravel-bed river. Freshwater Biology, 45: 57–73. Rendo´n M. A., Green A. J., Aguilera E. & P. A. (2008) Status, distribution and long-term changes in the waterbird community wintering in Don˜ ana, south– west Spain. Biological Conservation, 141: 1371 -88. Ringrose S., Vanderpost C., Matheson W., Wolski P., Huntsman-Mapila P., Murray-Hudson M. & Jellema A. (2007) Indicators of desiccation-driven change in the distal Okavango Delta, Botswana. Journal of Arid Environments, 68: 88-112. Roberts J. (2004) Floodplain forests and woodlands in the southern MurrayDarling Basin. Report for the Australian Conservation Foundatiton, Canberra. Roberts J. & Hale J. (2007) Lake Mokoan Littoral Vegetation:Monitoring Program. Jane Roberts, Canberra. Roberts J. & Marston F. (2000) Water regime of wetland and floodplain plants in the Murray Darling Basin: A source book of ecologcial knowledge. CSIRO Land and Water, Technical Report 30/00. October 2000, Canberra. 122 Robledano F., Esteve M. A., Farinós P., Carreño M. F. & Martínez-Fernández J. (2009) Terrestrial birds as indicators of agricultural-induced changes and associated loss in conservation value of Mediterranean wetlands Ecological Indicators, 10: 274-86. Robson B. J. & Matthews T. G. (2004) Drought refuges affect algal recolonization in intermittent streams. River Research and Applications, 20: 753–63. Rose P., Metzeling L. & Catzikiris S. (2008) Can macroinvertebrate rapid bioassessment methods be used to assess river health during drought in south eastern Australian streams? Freshwater Biology, 53: 2626–38. Roy A. H., Freeman M. C., Freeman B. J., Wenger S. J., Ensign W. E. & Meyer J. L. (2005) Investigating hydrologic alteration as a mechanism of fish assemblage shifts in urbanizing streams. Journal of the North American Benthological Society, 24: 656–78. Russell G. J., Bass Jr. O. L. & Pimm S. L. (2002) The effect of hydrological patterns and breeding-season flooding on the numbers and distribution of wading birds in Everglades National Park. Animal Conservation, 5: 185-99. Salter J., Morris K. & Boon P. I. (2008) Does salinity reduce the tolerance of two contrasting wetland plants, the submerged monocot Vallisneria australia and the woody shrub Melaleuca ericifolia, to wetting and drying? . Marine and Freshwater Research, 59: 291-303. Schmidt M., King E. A. & McVicar T. R. (2005) Development of a web-based data and product delivery system for the CSIRO AVHRR Time Series (CATS). (Series : CSIRO Atmospheric Research technical paper (Online) ; 66). Sheldon F. & Thoms M. (2006) Relationships between flow variability and macroinvertebrate assemblage composition: data from four Australian Dryland rivers. River Research and Applications, 22: 219–38. 123 Shivoga W. A. (2001) The influence of hydrology on the structure of invertebrate communities in two streams flowing into Lake Nakuru, Kenya. Hydrobiologia, 458: 121–30. Sims N. C. & Thoms M. C. (2002) What happens when flood plains wet themselves: vegetation response to inundation on the lower Balonne flood plain. In: The Structure, Function and Management Implications of Fluvial Sedimentary Systems. Proceedings of an international symposium Alice Springs, Australia. Skinner R., Sheldon F. & Walker K. F. (2001) Propagules in dry wetland sediments as indicators of ecological health: effects of salinity. Regulated Rivers: Research and Management, 17: 191–7. Suren A. M. & Jowett I. G. (2006) Effects of floods versus low flows on invertebrates in a New Zealand gravelbed river. Freshwater Biology, 51: 220727. Swiripek J. (2010) pers. comm. to Wayne Robinson. Murray Darling Basin Authority, Canberra. February 12. Thorburn P. J., Mensforth L. J. & Walker G. R. (1994) Reliance of Creek-side River Red Gums on Creek Water. Australian Journal of Marine and Freshwater Research, 45: 1439-43. Thorp J. H. & Mantovani S. (2005) Zooplankton of turbid and hydrologically dynamic prairie rivers. Freshwater Biology, 50: 1474–91. Tonkin J. D., Death R. G. & Joy M. K. (2009) Invertebrate drift patterns in a regulared river: dams, periphyton biomass or longitudinal patterns? River Research and Applications, 25: 1219–31. Uzarski D. G., Burton T. M. & Genet J. A. (2004) Validation and performance of an invertebrate index of biotic integrity for Lakes Huron and Michigan fringing wetlands during a period of lake level decline. Aquatic Ecosystem Health & Management, 7: 269–88. 124 Vaughan I. P., Diamond M., Gurnell A. M., Hall K. A., Jenkins A., Milner N. J., Naylor L. A., Sear D. A., Woodward G. & Ormerod S. J. (2009) Integrating ecology with hydromorphology: a priority for river science and management. Aquatic conservation: Marine and Freshwater Ecosystems, 19: 113-25. Verbesselt J., Hyndman R., Newnham G. & Culvenor D. (2010) Detecting trend and seasonal changes in satellite image time series Remote Sensing of Environment, 114: 106-15. Veselka IV W., Anderson J. T. & Kordek W. S. (2009) Using dual classifications in the development of avian wetland indices of biological integrity for wetlands in West Virginia, USA. Environmental Monitoring and Assessment. Walker K. (1985) A review of the ecological effects of river regulation in Australia. Hydrobiologia, 125: 111-29. Welcomme R. L. (1979) Fisheries ecology of floodplain rivers. Longman, London. Welcomme R. L., Christophe Bene C., Brown C. A., Arthington A., Dugan P., King J. M. & Sugunan V. (2006a) Predicting the Water Requirements of River Fisheries. In: Wetlands and Natural Resource Management (eds J. T. A. Verhoeven, B. Beltman, R. Bobbink and D. F. Whigham) pp. 123-54. SpringerVerlag, Berlin Heidelberg. Welcomme R. L., Winemiller K. O. & Cowx I. G. (2006b) Fish environmental guilds as a tool for assessment of ecological condition of rivers. River Research and Applications, 22: 377-96. Wen L., Ling J., Saintilan N. & Rogers K. (2009) An investigation of the hydrological requirements of River Red Gum (Eucalyptus camaldulensis) Forest, using Classification and Regression Tree modelling. Ecohydrology, 2: 143-55. 125 Whitfield A. K. & Taylor R. H. (2009) A review of the importance of freshwater inflow to the future conservation of Lake St Lucia. Aquatic conservation: Marine and Freshwater Ecosystems, 19: 838-48. Whitley J. R. & Campbell R. S. (1974) Some aspects of water quality and biology of the Missouri River. Transactions of the Missouri Academy of Science, 8: 60-72 (cited in Bunn and Arthington 2002). Woodland D. J. & Ward P. J. (1990) Fish communities in sandy pools of Magela Creek, Alligator Rivers Region. p. 81 pp. Supervising Scientist for the Alligator Rivers Region,. Young R. G., Matthaei C. D. & Townsend C. R. (2008) Organic matter breakdown and ecosystem metabolism: functional indicators for assessing river ecosystem health. Journal of the North American Benthological Society, 27: 605-25. Zhang Y., Malmqvist B. & Englund G. (1998) Ecological processes affecting community structure of blackfly larvae in regulated and unregulated rivers: a regional study. Journal of Applied Ecology, 35: 673-86. Zhou S., Tang T., Wu N., Fu X. & Cai Q. (2008) Impacts of a Small Dam on Riverine Zooplankton. International Review of Hydrobiology, 93: 297–311. 126