Organisms are relatively similar at a molecular level
advertisement

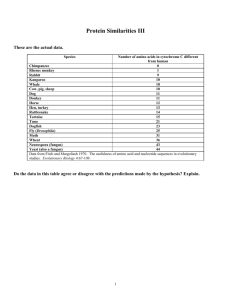
Protein Similarities I The molecule above is hemoglobin. Hemoglobin carries oxygen in the blood of mammals. Can you discern any secondary structures in the hemoglobin molecule? The part of the molecule represented by space-filling models is heme, a non-protein group that actually binds the oxygen. Suppose you found that the amino acid sequences of hemoglobin in a cat and a cow were very similar. How would you explain this? Please provide your answer on the separate sheet. A mutation in the gene coding for this protein could change the amino acid sequence. Are there some regions where you would expect a changed amino acid to cause the molecule not to work as well? Are there some changes that you would expect not to have much effect? Explain. Please provide your answer on the separate sheet. 1 Organisms are relatively similar at a molecular level. Indeed, many types of proteins are found in all organisms. Because of this, we should be able to compare the sequences of amino acids in their proteins to gain an understanding about their relationships. How much similarity in protein sequences would you expect between a whale and a fish? A whale and a dog? A dog and a shrimp? A shrimp and a bacterium? There are two types of similarity to be considered: analogy and homology. If the sequences in two species show similarity because they code for proteins (or regions of proteins) with similar functions, analogy would be the explanation. If the sequences show similarity because the two species share common ancestry, then the similarity is due to homology. Let's consider a protein that is found in almost all organisms. It's called cytochrome C. Recall that organisms all make ATP, an energy source for chemical reactions, by the process of cellular respiration. Obviously, the process is greatly simplified here. The energy in the sugar is in the form of highenergy electrons; it's released in a series of reactions in which the electrons are transferred to lower-energy compounds, step by step. Doing this requires some proteins that function as electron carriers; one of these is called cytochrome C. 2 This is the 3-D structure of cytochrome C. The light ribbon or tube represents the polypeptide chain. Note the binding site in the top of the molecule. Here the iron-containing molecule heme is bound in the binding site in cytochrome C. The iron atom in heme is what carries the electrons. The helical coils that you see in the Cytochrome C structure above are an example of what kind of structure? Please provide your answer on the separate sheet. The overall 3-D shape that this polypeptide folds up into is an example of what kind of structure? Please provide your answer on the separate sheet. Just about every organism known has cytochrome C, and its function is exactly the same in every organism. Thus, we can use this molecule to compare a lot of different organisms. We'll need to compare the primary structure of the protein -- that is, the sequence of amino acids that make it up. Here's a map of the amino acid sequence of cytochrome C: Cytochrome C is about 104 amino acids long (they vary slightly in different organisms). Of those amino acids, the 20 amino acids shown here as darker bands are the same in every organism known. The rest of them all show some variation among the organisms whose cytochrome c proteins have been sequenced. 3 In other words, those 20 amino acids represent a similarity among all eukaryotes (everything but bacteria) at least. What kind of similarity is this, homology or analogy? Remember that the function of cytochrome C is the same in all of these organisms, to carry electrons. Explain whether this similarity is homology or analogy, using the procedure below. Please provide your answer on the separate sheet. 4