There is always an equilibrium established
advertisement
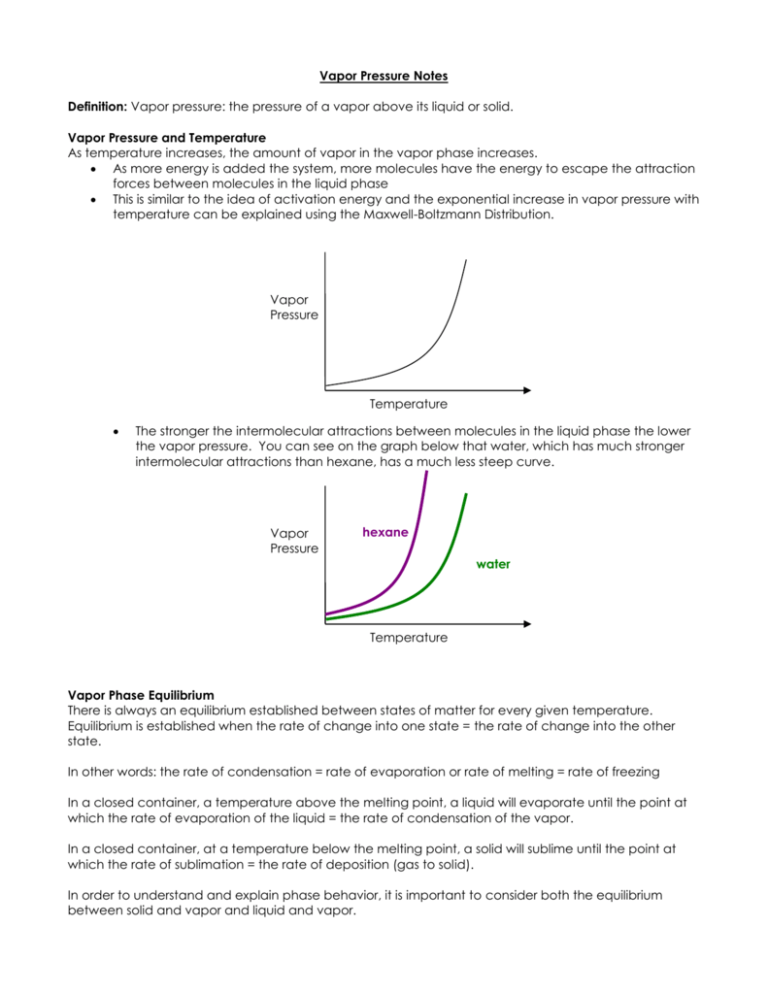
Vapor Pressure Notes Definition: Vapor pressure: the pressure of a vapor above its liquid or solid. Vapor Pressure and Temperature As temperature increases, the amount of vapor in the vapor phase increases. As more energy is added the system, more molecules have the energy to escape the attraction forces between molecules in the liquid phase This is similar to the idea of activation energy and the exponential increase in vapor pressure with temperature can be explained using the Maxwell-Boltzmann Distribution. Vapor Pressure Temperature The stronger the intermolecular attractions between molecules in the liquid phase the lower the vapor pressure. You can see on the graph below that water, which has much stronger intermolecular attractions than hexane, has a much less steep curve. Vapor Pressure hexane water Temperature Vapor Phase Equilibrium There is always an equilibrium established between states of matter for every given temperature. Equilibrium is established when the rate of change into one state = the rate of change into the other state. In other words: the rate of condensation = rate of evaporation or rate of melting = rate of freezing In a closed container, a temperature above the melting point, a liquid will evaporate until the point at which the rate of evaporation of the liquid = the rate of condensation of the vapor. In a closed container, at a temperature below the melting point, a solid will sublime until the point at which the rate of sublimation = the rate of deposition (gas to solid). In order to understand and explain phase behavior, it is important to consider both the equilibrium between solid and vapor and liquid and vapor. For a given temperature, above the melting point of a substance, the vapor pressure necessary for a solid and a vapor to maintain equilibrium is always higher than that of the liquid and vapor. Solid Vapor Pressure Liquid Melting point Temperature Therefore, when a solid exists at a temperature above the melting point, the solid will continue to sublime until that vapor pressure is reached. However, because the vapor pressure needed for equilibrium between a liquid and a vapor is less than for the solid, the vapor molecules (from the solid) will condense into a liquid so that the equilibrium between the vapor and liquid can be established. This continues until there is no more solid – only liquid and vapor. It appears that the solid has melted and this is also why solids cannot exist above their melting temperatures! As a way of illustrating the last point, let’s say that the solid is not happy until the amount of vapor = 8 and the liquid is not happy (at equilibrium) until the vapor = 6. Solid Vapor Time Vapor Liquid 0 Amount of Solid 10 Amount of Vapor 0 Amount of Liquid 0 1 4 6 0 2 3 7 0 3 2 7 1 4 1 7 2 5 0 7 3 6 0 6 4 What will happen Solid is not in equilibrium with vapor, according to LeChatelier – solid will vaporize to produce more vapor Solid is still not in equilibrium with vapor, according to LeChatelier, solid will continue to vaporize to produce more vapor. Neither liquid nor solid are in equilibrium, some vapor condenses to form a liquid, and more solid vaporizes Neither liquid nor solid are in equilibrium, some vapor condenses to form a liquid, and more solid vaporizes Neither liquid nor solid are in equilibrium, some vapor condenses to form a liquid, and more solid vaporizes Now all the solid is gone, and the liquid is still not in equilibrium with the vapor. So more vapor condenses. Now the liquid is in equilibrium with vapor. It appears that all the solid has melted and become liquid. **You might be asking yourself, why do 6 molecules change state in the first row, but only 1 molecule after that? The actual rate at which these changes happen doesn’t appear to be consistent in the table. It doesn’t matter. I hope you can see that if I was to make the rate more consistent, one molecule each time, the end result would be the same, but the amount of space the table takes up would be a lot more. What happens at a temperature below the melting point? In this situation the vapor pressure of the solid is less than that of the liquid. A similar case can be established which shows why it appears that all of a liquid, at a temperature below the melting point, freezes and why liquids don’t exist below their melting points. In this situation, because we are considering a system at a temperature lower than the melting point. The amount of vapor needed for a solid/vapor equilibrium will be lower than that of a liquid/vapor equilibrium. Amount of vapor necessary for liquid/vapor equilibrium = 12, Amount of vapor needed for a solid/vapor equilibrium: 8 Liquid Vapor Time Vapor Solid Amount of Vapor 0 Amount of Solid What will happen 0 Amount of Liquid 15 0 1 7 8 0 2 6 9 0 3 5 9 1 4 4 9 2 5 0 9 6 6 0 8 7 Liquid is not in equilibrium with vapor, according to LeChatelier – liquid will vaporize to produce more vapor Liquid is still not in equilibrium with vapor, according to LeChatelier, liquid will continue to vaporize to produce more vapor. Neither liquid nor solid are in equilibrium, some vapor deposits in order to lower the vapor pressure, and more liquid vaporizes. Neither liquid nor solid are in equilibrium, some vapor deposits in order to lower the vapor pressure, and more liquid vaporizes. Neither liquid nor solid are in equilibrium, some vapor deposits in order to lower the vapor pressure, and more liquid vaporizes. Now all the liquid is gone, and the solid is still not in equilibrium with the vapor. So more vapor undergoes deposition. Now the solid is in equilibrium with vapor. It appears that all the liquid has frozen and become solid. Definition: Melting point: the temperature at which the equilibrium vapor pressure of a solid and a liquid are equal. Normal melting point: the temperature at which the equilibrium vapor pressure of a solid and liquid are equal and the total pressure = 1atm. Solid Vapor Pressure Liquid Melting point Temperature Boiling vs. Evaporation Definition: Boiling point: the temperature at which the vapor pressure of a liquid = atmospheric pressure. Normal boiling point: the temperature at which the vapor pressure = 1 atm. Boiling is characterized by the formation of bubbles. This occurs when the pressure of the vapor can overcome the surrounding pressure and form a bubble in the liquid. If the vapor pressure is less than the surrounding pressure, a bubble cannot form. The conversion between liquid to vapor occurs in the middle of the liquid. While, with evaporation, the conversion from liquid to gas only occurs at the surface. Phase Diagrams A phase diagram shows how the state of matter and the equilibrium position between two states of matter changes with temperature and pressure. Phase Diagram for Water Liquid Solid-liquid Line Pressure (atm) Liquid-Vapor LIne 1.00 Solid 0.0060 Gas Solid –Vapor LIne 0.00 100.0 Temperature (C) Things to note The solid-liquid line shows the melting point at different pressures. This is where the solid and liquid are in equilibrium. The liquid-vapor line shows the boiling point at different pressures. This is where the liquid and vapor are in equilibrium. As pressure increases, the boiling point increases. Remember that boiling point is defined as the temperature at which the vapor pressure = surrounding pressure. Therefore, if you increase the surrounding pressure, vapor pressure must increase before boiling can occur. As pressure increases, the melting point decreases. This is true for water, but not other substances. It results from the crystal structure formed by H 2O molecules in ice. The crystal structure results in a less dense form than liquid H 2O, with larger spaces present between the H2O molecules in the solid compared to the spacing between molecules in the liquid phase. So when increasing pressures, the molecules of water get closer together. Molecules closer together = liquid. Vapor Pressure of Mixtures Non-volatile vs. Volatile Non-volatile substances: substances that have very low vapor pressures at a given temperature. They don’t readily evaporate and are often solids at room temperature. When a solid is mixed with a solvent, the vapor pressure of the solvent lowers. This is an observable phenomenon. By why does it happen? The rate at which molecules of solvent can escape is lowered. The rate at which molecules escape to the gas phase is related to the total volume of solvent. When some of the volume is taken up by solute particles, the rate of escape decreases. This changes the equilibrium constant and equilibrium position. Raoult’s Law says that the vapor pressure of a mixture between a solvent and non-volatile solute is = to the product of the mole fraction of the solvent and the vapor pressure of the pure solvent. Psoln = Xsolvent Psolvent Because vapor pressure depends on the amount of solute and solvent and not what the solute and solvent are, Raoult’s Law can be used to o find molar mass of an uknown o used to find out if the solute dissociates when dissolved in the solvent When two volatile solutions are mixed, both vapors contribute to the total pressure of the vapor phase. Raoult’s Law changes to: Ptotal = PA + PB = XAPA0 + XBPB0, 0 where the P means the vapor pressure of the pure substance. Total Pressure of Solution Vapor Pressure Partial Pressure of A Partial Pressure of B Mole Fraction Non-Ideal Behavior When two solutions mix, the intermolecular attraction between the molecules can result in nonideal behavior. In other words, the rate at which molecules escape to the gas phase can also be affected by the attractions between the molecules. Colligative Properties We stated above that the melting point is defined by vapor pressure. Boiling point is also defined in terms of vapor pressure. We also stated that by adding a solute, the vapor pressure is lowered. Therefore, when a solute is added, the melting point and boiling point also change. Solid Vapor Pressure Liquid Mixture New Melting point Melting point Temperature The phase diagram for a solvent changes slightly when considering mixtures. Pressure (atm) 1.00 0.0060 -1.5 0.00 100.0 105.0 Temperature (C) Notice that the normal boiling and melting/freezing point change. Freezing point gets lower o At the freezing point, the rate of the solvent freezing must equal the rate of solvent melting. o The solute particles get in the way of the solvent freezing at the normal freezing point so the rate of freezing slows. It is not until a lower temperature is reached and the rate of solvent melting is slowed that the two rates become equal. Boiling point gets higher o Since the boiling point occurs when the vapor pressure = surrounding pressure, and we know that vapor pressure is reduced in the presence of a solute, the temperature must be increased in order to reach the boiling point. Equation: T = Kb/f m Molality of solution: mol of solute/ kg solvent Kb, molal boiling point elevation constant (unique to a solvent) Kf, molal freezing point depression constant (unique to a solvent) T, change in boiling/freezing temperature from pure solvent








