Cell ID - Nature
advertisement

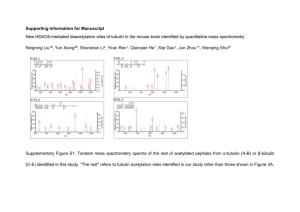
SUPPLEMENTARY DATA Suppl. Scheme 1: Chemical synthesis of Red-Br-nos Red-Br-nos((S)-3-(R)-9-bromo-5-(4,5-dimethoxy-1,3-dihydroisobenzofuran-1-yl)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinoline) Experimental: General: 1H NMR and 13 C NMR spectra were measured in CDCl3 on INOVA 400 NMR spectrometer. All proton NMR spectra were recorded at 400 MHz. All carbon NMR spectra were recorded at 100 MHz and were referenced with 77.27 ppm resonance of residual chloroform. Abbreviations for signal coupling are as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. Infrared spectra were recorded on sodium chloride discs on Mattson Genesis II FT-IR. High resolution mass spectra were collected on Thermo Finnigan LTQ-FT Hybrid mass spectrophotometer using 3-nitrobenzyl alcohol, in some cases with addition of LiI as a matrix. Melting points were determined using a Thomas Hoover melting point apparatus and were uncorrected. All reactions were conducted in oven-dried (125°C) glassware under an atmosphere of dry argon. All common reagents and solvents were obtained from commercial suppliers and used without further purification unless otherwise indicated. Solvents were dried by standard methods. The reactions were monitored by thin layer chromatography (TLC) using silica gel 60 F254 (Merck) precoated glass sheets. Flash chromatography was carried out on standard grade silica gel (230-400 mesh). Synthesis of reduced bromonoscapine (Red-Br-nos) 2 was carried out starting from noscapine (NOS) in two steps. The first step involved bromination of noscapine molecule using Nbromosuccinimide (NBS) in presence of triethylamine in DMF at 50°C for 4h to give 9bromonoscapine 1. In the next step, the carbonyl moiety of the lactone ring of 1 was reduced 1 efficiently by triethylsilane in presence of a moderately strong lewis acid, borontrifluoride diethyletherate to produce the target compound 9-bromo-5-(4,5-dimethoxy-1,3- dihydroisobenzofuran-1-yl)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinoline 2. This new method to synthesize Red-Br-Nos is relatively better than the one described earlier1, as it avoids the use of HBr (a strong acid) and pH adjustment of the reaction mixture using alkali. Furthermore, it gives better yield in the second step (carbonyl reduction). (S)-3-((R)-9-bromo-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin5-yl)-6,7-dimethoxyisobenzofuran-1(3H)-one (1): Noscapine (1.0 g, 2.42 mmol) and Nbromosuccinimide (1.7 g 4.11 mmol) were dissolved in 5.0 ml of anhydrous DMF followed by the addition of triethylamine (0.67 ml, 4.84 mmol). This reaction mixture was stirred at 50°C for 8h. After TLC control the reaction mixture was concentrated and residue was taken in chloroform (20 ml) and washed with 1M sodium bicarbonate solution followed by water and dried over sodium sulfate. The organic layer was then concentrated under vacuum to get 2 as yellowish solid. MS (ESI/tandem mass spectrometry): [M+H] = 493 (S)-3-(R)-9-bromo-5-(4,5-dimethoxy-1,3-dihydroisobenzofuran-1-yl)-4-methoxy-6-methyl5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinoline (2): 9-bromonoscapine 2 (0.5 g, 1.21 mmol) was taken in 5 ml of dry THF and cooled to 0°C. To this solution was added borontrifluoride etherate (1.0 ml) and triethylsilane (0.5 ml, 3.15 mmol). The resulting mixture was stirred magnetically at 60°C, over an oil bath for 4h. After TLC control the reaction mixture was concentrated under reduced pressure and the residue obtained was taken in ethyl acetate (25 ml). The organic layer was washed with 1 M sodium bicarbonate solution, brine and water respectively and dried over sodium sulfate. Solvent was removed in vacuo to get crude product which was chromatographed over flash silica (CH2Cl2 and MeOH, 98:2) to get title compound 2 as yellowish solid. MS (ESI/tandem mass spectrometry): [M+H] = 480 2 Cell ID Group ID #Daughters 8 S1 2 8 S2 1 8 D1 2 8 D2 1 9 M1 0 9 M2 1 9 D1 2 9 D2 1 9 D3 1 Suppl. Table 1: Sample records for representative cells 8 and 9. The first four records define cell 8, indicating that there are four physically distinct groups of centrioles, as follows: S1 and S2 indicate that there are two groups, each containing two mothers, one sharing two daughters and the other sharing a single daughter; there are also two distinct de novo groups, one with two daughters and another with an isolated daughter; no isolated mother groups are present. In cell 9 there is an isolated mother centriole with no associated daughters, another mother with one associated daughter, and three distinct de novo groups with differing numbers of daughters. 3 Suppl. Fig. 1: Attenuation of ROS upon a 4h tiron treatment prior to Red-Br-nos exposure for 12h showed a marked reduction in the proportion of cells with γ-H2AX foci as compared to drug treatment alone. Quantitation of the percentage of cells harboring γ-H2AX foci for the indicated treatments is shown as a bar-graphical representation. Suppl. Fig. 2: Binding of Red-Br-nos to free tubulin and its effect on tubulin tertiary structure. Tubulin was isolated from bovine brain by two cycles of polymerization and depolymerization without glycerol. MAP-free tubulin was purified from MAP-rich tubulin by phosphocellulose chromatography, was drop-frozen in liquid nitrogen, and stored at -70ºC until used2. All fluorescence measurements were performed using a Perkin Elmer LS 50B spectrofluorometer equipped with a constant temperature water-circulating bath. A 0.3cm pathlength quartz cuvette was used for the measurements. A. Time-dependent binding of Red-Brnos to tubulin. The binding of Red-Br-nos to soluble bovine brain tubulin was studied by measuring the quenching of the intrinsic tryptophan fluorescence of tubulin3. Red-Br-nos (100 µM) was incubated with 1 µM tubulin in PEM buffer for 45 min at 25°C. The relative intrinsic fluorescence intensity of tubulin was monitored at 335 nm at an excitation wavelength of 295 nm either immediately after addition of Red-Br-nos or at 30th and 60th min after the drug addition. The fluorescence emission intensity of Red-Br-nos at 295 nm was negligible. Fluorescence data was corrected for the inner filter effect using the Lakowicz equation Fcorrected = Fobserved antilog [(Aex + Aem)/2], where Aex is the absorbance at the excitation wavelength and Aem is the absorbance at the emission wavelength. The graph represents one of the two independent experiments. B. Effect of Red-Br-nos on 1-anilinonaphthalene-8-sulfonic acid (ANS) binding 4 to tubulin. ANS binds to hydrophobic surfaces on proteins and thus is used as a probe to study perturbation of tertiary structure of proteins by ligands. The binding of ANS to tubulin was measured using methods described previously4. Tubulin (2 µM) was incubated with Red-Br-nos (0-200 µM) for 20 min in PEM buffer at 25ºC. ANS (30 µM) was added to the mixture and were incubated for 20 min at 25ºC. The samples were excited at 380 nm and the emission readings were recorded at 470 nm. The experiment was performed three times. Data, mean SD. Suppl. Fig. 3: Immunofluorescence micrographs of control untreated PC-3 cells showing progressive cell-cycle stages. γ-tubulin is shown in green, microtubules in red and DNA (DAPI) in blue. Interphase cells show a single centrosome and metaphase cells show the expected bipolar spindle configuration. Scale bar = 10 m Suppl. Fig. 4: Bar-graphical quantitation showing the percentage of PC-3 cells that display bipolar or multipolar spindle phenotype with increasing duration of Red-Br-nos exposure (10 µM). Analysis was based on the visual determination of phenotypes from randomly selected fields totaling to at least 200 mitotic cells under a confocal microscope using a 63X objective (NA 1.4). 5 Suppl. Fig. 5: Red-Br-nos is much more active than the parent noscapine in inhibiting the proliferation of various human cancer cells. The panel of 60 human tumor cell lines is organized into subpanels representing leukemia, non-small cell lung, colon, CNS, melanoma, ovarian renal, prostate and breast cancer lines. Cells were treated with noscapine and Red-Br-nos at increasing gradient concentrations for 48h. The IC50 values, which represent the drug concentration needed to prevent cell proliferation by 50% was then measured using an in vitro Sulforhodamine B assay. Panels show bar-graphically the comparison of IC50 values of noscapine (black bars) and Red-Br-nos (grey bars) for cancer cell lines from various tissue origins. 6 Suppl. Fig. 6: Red-Br-nos induces ROS-mediated autophagy at 10 µM. A. Immunoblot analysis of LC3-II expression levels in lysates from PC-3 cells treated with 10 μM Red-Br-nos for the indicated time points. β-Actin was used as a loading control. B. Immunofluorescence microscopy of DCF, DHE and acridine orange-stained PC-3 cells treated for 24 h with DMSO (control) or 10 μM Red-Br-nos. Panel i shows microscopic visualization of DCF fluorescence in control and drug-treated PC-3 cells. Panel ii shows microscopic visualization of EtBr (EB) fluorescence in control and drug-treated PC-3 cells. Panel iii represents ncrease in number of cells with AO accumulating acidic vesicular organelles (orange-red fluorescence) in Red-Brnos-treated cells as compared to control. Green profile depicts control cells that were stained with AO and the red profile shows drug-treated cells with increased AO fluorescence, indicative of numerous red AVOs. 7 Supplementary movie-1: Time-lapse recordings of MDA-MB-231 cells treated with 10 µM Red-Br-nos for 9 hours. Cells clearly show induction of several new green dots corresponding to numerous centrioles. Supplementary movie-2: Time-lapse recordings of MDA-MB-231 cells stably transfected with GFP-centrin for 12 hours. Typical centrosomes with 2 centrioles are evident and there is no appearance of numerous or abnormal centrioles References 1. Zhou J, Gupta K, Aggarwal S, Aneja R, Chandra R, Panda D et al. Brominated derivatives of noscapine are potent microtubule-interfering agents that perturb mitosis and inhibit cell proliferation. Mol Pharmacol 2003; 63(4): 799-807. 2. Yenjerla M, LaPointe NE, Lopus M, Cox C, Jordan MA, Feinstein SC et al. The neuroprotective peptide NAP does not directly affect polymerization or dynamics of reconstituted neural microtubules. J Alzheimers Dis 2010; 19(4): 1377-86. 3. Lopus M, Oroudjev E, Wilson L, Wilhelm S, Widdison W, Chari R et al. Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules. Mol Cancer Ther 2010; 9(10): 2689-99. 4. Lopus M, Panda D. The benzophenanthridine alkaloid sanguinarine perturbs microtubule assembly dynamics through tubulin binding. A possible mechanism for its antiproliferative activity. FEBS J 2006; 273(10): 2139-50. 8