Taxon - Proceedings of the Royal Society B
advertisement

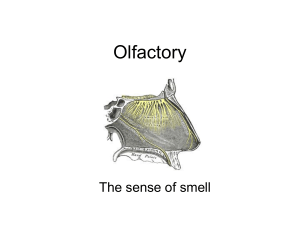
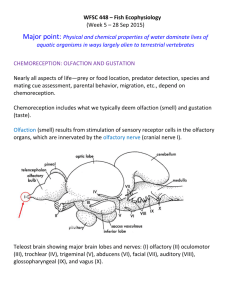
Electronic Supplementary Material - Zelenitsky et al. Relationships between olfactory ratio and general habits in birds Early work on olfactory ratios in birds revealed associations between olfactory ratio and foraging, diet, nesting, or breeding habits (Cobb 1960; Bang 1971; Bang & Wenzel 1985), although lacked rigorous statistical analyses. A subsequent study (Healy & Guilford 1990) attempted to examine statistical correlations between olfactory bulb length (not olfactory ratio) and general habits (activity timing, diet, nest type, development, nest dispersion, migratory behaviour) of birds and found a significant correlation only between olfactory bulb length and activity timing (nocturnality and crepuscularity). Although Healy & Guilford (1990) conducted statistical analyses of olfactory bulb length in relation to general habits of birds, they did not truly test the associations between olfactory bulb size and ecological parameters established by Cobb (1960) and Bang (1971). Even though Healy & Guilford (1990) may have used the olfactory bulb lengths of species reported by Cobb (1960) and Bang (1971), they averaged these values to the family level to address the phylogenetic relationships of taxa in their analyses, an approach now considered invalid (Martins & Garland 1991). Furthermore, they used different parameters (e.g., development, migratory behavior) and different groupings within a given parameter from those established by Bang (1971). For example, Healy & Guilford’s (1990) diet categories (fruit, foliage, invertebrates, lower vertebrates, plant material + invertebrates, invertebrates + lower vertebrates + higher vertebrates + carrion) were distinctly different from Bang’s (1971) diet categories (carnivorous + piscivorous, vegetarian + frugivorous, insectivorous, granivorous, omnivorous). Therefore, the fact that Healy & Guilford (1990) used different parameters (and different categories within these parameters) than those used by Bang (1971) could explain, in part, the difference in correlation/relationships between olfactory bulb size and ecological parameters between these two studies. Data Analysis and Acquisition Justification for use of olfactory ratios in theropods: Olfactory ratios rather than volumes were used to quantify olfactory bulb size in extinct theropods because: 1) volumetric data cannot be obtained from specimens that are incomplete or the sphenethmoid is unossified (in maniraptoriforms); and 2) olfactory ratios have been used widely for birds in the literature, and are the standard for the quantification of olfactory bulb size in behavioural studies (e.g., Bang & Wenzel, 1985; Healy & Guilford 1990; Verheyden & Jouventin 1994; Steiger et al. 2008). Changes in cerebral hemisphere size through theropod evolution (Larsson et al. 2000; Larsson 2001) could be used as an argument to invalidate the use of olfactory ratios based on the assumptions that: 1) changes were restricted to the cerebral hemispheres and were not mirrored in the olfactory apparatus; and 2) the same cerebral hemisphere dimension (e.g. length) is compared among all taxa, such that evolutionary changes in shape could be missed. Whereas there is no published study supporting or falsifying the first assumption, the use of the greatest cerebral diameter, regardless of its dimension (length, width, and depth), allows for some of the cerebral morphological differences between taxa to be accounted. As such, olfactory ratios can reasonably be used as a proxy for the importance of olfaction relative to other senses irrespective of whether changes occur in olfactory bulb size (i.e numerator of ratio) or in cerebral hemisphere size (i.e. denominator of ratio). Olfactory ratio calculations: Olfactory ratios were obtained from absolute measurements of braincases, endocasts, or from measurements of CT-scan slices. Ratios were also determined from illustrations of three-dimensional digital reconstructions of the endocranial cavity. For the latter, linear dimensions were acquired from the same aspect (i.e. dorsal or lateral view) of the endocast without conversion to original dimensions (in cm) in order to avoid errors due to imprecision of scale bars. Thus, although the true dimensions of the endocranial cavity were not determined for illustrations, the proportion between the olfactory and cerebral regions is accurate. Olfactory ratios obtained from illustrations produced results comparable to those obtained from measurements on specimens/casts of related taxa, thus justifying their validity. The source/specimen number of the olfactory ratio data is indicated under “catalogue number” in Table S1. Olfactory ratio calculations for some specimens require explanation due to specimen preservation. The olfactory ratio of one of the Tyrannosaurus rex endocasts (AMNH 5029) may be underestimated because the depth of the olfactory bulb is uncertain due to a missing sphenethmoid. The olfactory ratios of Albertosaurus sarcophagus and Gorgosaurus libratus were derived from fronto-parietal complexes that preserve neither the depth of the olfactory bulbs nor the entire length of the cerebral hemispheres; consequently, these olfactory ratios should be considered maximum values. In Troodon formosus, the insertion site of the mesethmoid and olfactory bulb impressions on the frontals reveal that the olfactory bulbs occupied approximately 60% of the total length of the olfactory fossa; consequently the length of the olfactory fossa was adjusted proportionally to reflect the true length of the olfactory bulbs. In Saurornitholestes langstoni, the posterior portion of the endocast is unknown; consequently, its olfactory ratio should be considered an approximation. Independent contrast methods: A branch length of one was assigned to the phylogeny for independent contrasts (Figure S1). Albertosaurus and Gorgosaurus were not included in the independent contrast analysis because of the uncertainty of their olfactory ratios and body masses. For taxa in which several individuals were studied, specimens of Allosaurus (UUVP 5961), Tyrannosaurus rex (FMNH PR 2081), and Troodon (TMP 86.36.4) were considered because either their body mass estimates were the least subject to uncertainty (Allosaurus and Tyrannosaurus) or their olfactory ratio was close to the mean value of all individuals (Troodon). Least-squares regression of the standardized, positivized independent contrasts of olfactory ratio and body mass (slope = 0.1237, r = 0.87) is presented (Figure S2). To reconstruct the ancestral state of the common ancestor of Dilong and tyrannosaurids, the tree was rerooted to place that node at the base of the tree and the number of branch lengths deleted in the process was added to the overlying branch, following the method of Garland et al. (1999). Figure S1. Phylogeny used to calculate phylogenetic independent contrasts of olfactory ratio on body mass for 19 theropod species. The topology of the tree was obtained from Holtz & Osmólska (2004) and Kobayashi & Barsbold (2005). Numbers indicate nodes and tips. Figure S2. Relationship between phylogenetic independent contrasts of the olfactory ratio on body size for 19 theropod species. Numbers refer to the nodes in Figure S1. Institutional abbreviations for Table S1. AMNH, American Museum of Natural History, New York City, NY; BMNH, British Museum of Natural History, London, UK; FMNH PR, Field Museum of Natural History, Chicago, IL; GIN, Paleontological Center of Mongolia, Ulaan Bataar, Mongolia; IGM, Institute of Geology, Mongolian Academy of Sciences, Ulaan Bataar, Mongolia; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China; KUVP, Kansas University Natural History Museum, Lawrence, KS; MUCPv-CH, Museo de la Universidad Nacional del Comahue, El Chocón collection, Neuquén; MWC, Museum of Western Colorado, Fruita, CO; NMC, Canadian Museum of Nature, Ottawa; OMNH, Oklahoma Museum of Natural History, Norman, OK; PIN, Palaeontological Institute, Moscow; ROM, Royal Ontario Museum, Toronto, Ontario; SGM, Ministère de l’Énergie et des Mines, Rabat, Morocco; TMP, Royal Tyrrell Museum of Palaeontology, Drumheller, Alberta; UF, University of Florida, Gainesville, FL; UUVP, University of Utah Museum of Natural History, Salt Lake City, UT; ZPAL, Institute of Paleobiology of the Polish Academy of Sciences, Warsaw, Poland. Table S1. Calculated olfactory ratios and body masses of theropod and crocodylian specimens studied. Source is provided for specimens measured and when body mass is based on published femur length or different specimens from endocasts. Body mass estimates for theropods are based on femur length, following Christiansen and Fariña’s (2004) method, except for Majungasaurus where a published estimate based on femur circumference is used; body mass estimates for Alligator are based on skull length following Farlow et al.’s (2005) method. Asterisk indicates maximum values as it was not possible to determine the full length of the cerebral hemispheres and depth of the olfactory bulbs in Albertosaurus and Gorgosaurus because measurements were made on frontoparietal complexes. Taxon Allosauroidea Allosaurus fragilis Acrocanthosaurus atokensis Carcharodontosaurus saharicus Giganotosaurus carolinii Ceratosauria Ceratosaurus magnicornis Majungasaurus crenatissimus Tyrannosauroidea Dilong paradoxus Albertosaurus sarcophagus Catalogue number/specimen details Olfactory bulb/Cerebral hemisphere greatest diameters Olfactory ratio (%) Cast of UUVP 294 UUVP 5961 (Franzosa, 2004: fig. 28) OMNH 10146 (Franzosa and Rowe, 2005: fig. 2) SGM-Din 1 (endocast) Length/Depth 51.6 50 Femur length (mm) Body mass (kg) 860 (UUVP 6000 in Paul, 1988) 1468.77 Length/Depth 58.1 ~1153 (Stovall and Langston, 1950) 3777.58 Depth/Length 56 ~1450 (Sereno et al., 1996) 7905.47 MUCPv-CH-1 (CT scan slices) Length/Length 57.7 1430 (Sereno et al., 1996) 7559.49 MWC 1 (Sanders and Smith, 2005: fig. 13) FMNH PR 2100 (Sampson and Witmer, 2007: fig. 18) Length/Length 48.1 538.86 Length/Length 48.3 630 (Madsen and Welles, 2000) Sampson and Witmer (2007) IVPP V14243 (CT scan slices) TMP 81.9.1 (frontoparietal) Length/Length 27 9.69 Length/Length* 71* Length/Length* 68.5* 181 (Xu et al., 2004) 1020 (ROM 807, Currie, 2003) 1040 (NMC 2120, Currie, 2003) 970 (PIN 551-3, Paul, 1988) Gorgosaurus libratus TMP 67.14.3 (frontoparietal) Tarbosaurus bataar Cast of PIN 46104 Depth/Length 65.1 Tyrannosaurus rex AMNH 5029 (subadult endocast) FMNH PR 2081 Depth/Length 66.5 71 980 (AMNH 30564, Erickson et al., 2004) 1321 (Brochu, 1130 2545.13 2709.45 2164.60 2237.33 5855.30 (Brochu and Ketcham, 2003: digital endocast) Ornithomimosauria Garudimimus brevipes 2003) GIN 100/13 (CT scan slices) Length/Length 28.8 Ornithomimus edmontonensis TMP 95.110.1 (CT scan slices) Length/Length 31.4 Dromiceiomimus brevitertius NMC 12228 (endocast) Length/Length 29.4 468 (Russell, 1972) 206.79 Struthiomimus altus TMP 90.26.1 (CT scan slices) Length/Length 32.5 486 277.97 IGM 100/978 (Franzosa, 2004: fig. 30) Depth/Length 31.5 ~405 (IGM 100/1004, G.M. Erickson personal communication 2008) 129.78 TMP 74.10.5 (endocast) Length/Length 34.8 214 16.62 KUVP 129737 (Burnham, 2004: fig. 3.2) GIN 100/24 (CT scan slices) Length/Length 28.5 118 (Burnham, 2004) 2.44 Length/Length 35.7 200 (GIN 100/25, Paul, 1988) 13.36 TMP 79.8.1 TMP 86.36.4 NMC 12340 AMNH 6174 (frontoparietals) Length/Length 33.2 33.5 32.6 33 320 (MOR 748, D.J. Varricchio personal communication 2008) 60.76 BMNH 37001 (Dominguez Alonso et al., 2004: fig. 3) Length/Length 17.1 60.5 (Paul, 1988) 0.28 Oviraptoridae Citipati osmolskae Dromaeosauridae Saurornitholestes langstoni Bambiraptor feinbergi Velociraptor mongoliensis Troodontidae Troodon formosus Aves Archaeopteryx lithographica Alligatoridae Alligator mississippiensis UF 98341 UF 105541 UF 87885 Length/Depth 49.8 54.3 55.1 371 (Kobayashi and Barsbold, 2005) 426 Skull length (mm) 434 367.5 311.5 97.84 152.74 161.60 90.60 50.90 (braincases) References Bang, B. G. 1971. Functional anatomy of the olfactory system in 23 orders of birds. Acta Anat. 79, 1-76. Bang, B. G. & Wenzel, B. M.. 1985. Nasal cavity and olfactory system. In Form and function in birds (eds A. S. King & J. McLelland), pp. 195-225. New York, NY: Academic Press. Brochu, C. A. 2003. Osteology of Tyrannosaurus rex: Insights from a nearly complete skeleton and high-resolution computed tomographic analysis of the skull. J. Vert. Paleont. 22 (Suppl., Memoir 7), 1-138. Brochu, C. A. & Ketcham, R. A. 2003. Computed tomographic analysis of the Tyrannosaurus rex skull. Society of Vertebrate Paleontology Memoir 7:CD-ROM. Burnham, D. A. 2004. New information on Bambiraptor feinbergi (Theropoda: Dromaeosauridae) from the Late Cretaceous of Montana. In Feathered dragons: Studies on the transition from dinosaurs to birds (eds P. J. Currie, E. B. Koppelhus, M. A. Shugar & J. L. Wright), pp. 67-111. Bloomington, IN: Indiana University Press. Christiansen, P. & Fariña. R. A. 2004. Mass prediction in theropod dinosaurs. Hist. Biol. 16, 8592. Cobb, S. 1960. A note on the size of the avian olfactory bulbs. Epilepsia 1, 394-402. Currie, P. J. 2003. Allometric growth in tyrannosaurids (Dinosauria: Theropoda) from the Upper Cretaceous of North America and Asia. Can. J. Earth Sci. 40, 651-665. Dominguez Alonso, P. D., Milner, A. C., Ketcham, R. A., Cookson, M. J. & Rowe ,T. B. 2004. The avian nature of the brain and inner ear of Archaeopteryx. Nature 430, 666-669. Erickson, G. M., Makovicky, P. J., Currie, P. J., Norell, M. A., Yerby, S. A. & Brochu, C. A.. 2004. Gigantism and comparative life-history parameters of tyrannosaurid dinosaurs. Nature 430, 772-775. Farlow, J. O., Hurlburt, G. R., Elsey, R. M., Britton, A. R. C. & Langston, Jr., W. 2005. Femoral dimensions and body size of Alligator mississippiensis: Estimating the size of extinct mesoeucrocodylians. J. Vert. Paleont. 25, 354-369. Franzosa, J. W. 2004. Evolution of the brain in Theropoda (Dinosauria). Ph.D. dissertation, University of Texas at Austin, Austin, Texas. Franzosa, J. & Rowe ,T. 2005. Cranial endocast of the Cretaceous theropod dinosaur Acrocanthosaurus atokensis. J. Vert. Paleont. 25, 859-864. Garland, T., Jr., Midford, P. E. & Ives, A. R. 1999. An introduction to phylogenetically based statistical methods, with a new method for confidence intervals on ancestral values. Amer. Zool. 39, 374-388. Healy, S., & Guilford, T. 1990. Olfactory-bulb size and nocturnality in birds. Evolution 44, 339346. Holtz, T. J., Jr. & Osmólska, H. 2004. Saurischia. In The Dinosauria, 2nd edition (eds D. B. Weishampel, P. Dodson & H. Osmólska), pp. 21-24. Berkeley, CA: University of California Press. Kobayashi, Y. & Barsbold, R. 2005. Reexamination of a primitive ornithomimosaur, Garudimimus brevipes Barsbold, 1981 (Dinosauria:Theropoda) from the Late Cretaceous of Mongolia. Can. J. Earth Sci. 42, 1501–1521. Larsson, H. C. E. 2001. Endocranial anatomy of Carcharodontosaurus saharicus (Theropoda: Allosauroidea) and its implications for theropod brain evolution. In Mesozoic vertebrate life (eds D. H. Tanke and K. Carpenter), pp. 19-33. Bloomington, IN: Indiana University Press. Larsson, H. C. E., Sereno, P. C. & Wilson, J. A. 2000. Forebrain enlargement among nonavian theropod dinosaurs. J. Vert. Paleont. 20, 615-618. Martins, E. P. & Garland, T., Jr. 1991. Phylogenetic analyses of the correlated evolution of continuous characters: a simulation study. Evolution 45, 534-557. Madsen, J. H., Jr. & Welles, S. P. 2000. Ceratosaurus (Dinosauria, Theropoda), a revised osteology. UT Geol. Surv. Misc. Publ. 00-2, 1-80. Paul, G. S. 1988. Predatory dinosaurs of the world. New York, NY: Simon and Schuster. Russell, D. A. 1972. Ostrich dinosaurs from the Late Cretaceous of Western Canada. Can. J. Earth Sci. 9, 375-402. Sampson, S. D. & Witmer, L. M. 2007. Craniofacial anatomy of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. J. Vert. Paleont. 27 (Suppl., Memoir 8), 32-102. Sanders, R. K. & Smith, D. K.. 2005. The endocranium of the theropod dinosaur Ceratosaurus studied with computed tomography. Acta Palaeontol. Pol. 50, 601-616. Sereno, P. C., Dutheil, D. B., Iarochene, M., Larsson, H. C. E., Lyon, G. H., Magwene, P. M., Sidor, C. A., Varricchio, D. J. & Wilson, J. A. 1996. Predatory dinosaurs from the Sahara and Late Cretaceous faunal differentiation. Science 272, 986–991. Steiger, S. S., Fidler, A. E., Valcu, M. & Kempenaers, B. 2008. Avian olfactory receptor gene repertoires: evidence for a well-developed sense of smell in birds? Proc. R. Soc. B, doi: 10.1098/rspb.2008.0607. Stovall J. W. & Langston, W., Jr. 1950. Acrocanthosaurus atokensis, a new genus and species of Lower Cretaceous Theropoda from Oklahoma. Am. Midl. Nat. 43, 696-728. Verheyden, C. & Jouventin, P. 1994. Olfactory behavior of foraging procellariiforms. The Auk 111, 285-291. Xu, X., Norell, M. A., Kuang, X., Wang, X., Zhao, Q. & Jia, C. 2004. Basal tyrannosauroids from China and evidence for protofeathers in tyrannosauroids. Nature 431, 680-684.