תרגיל בית מס` 2
advertisement
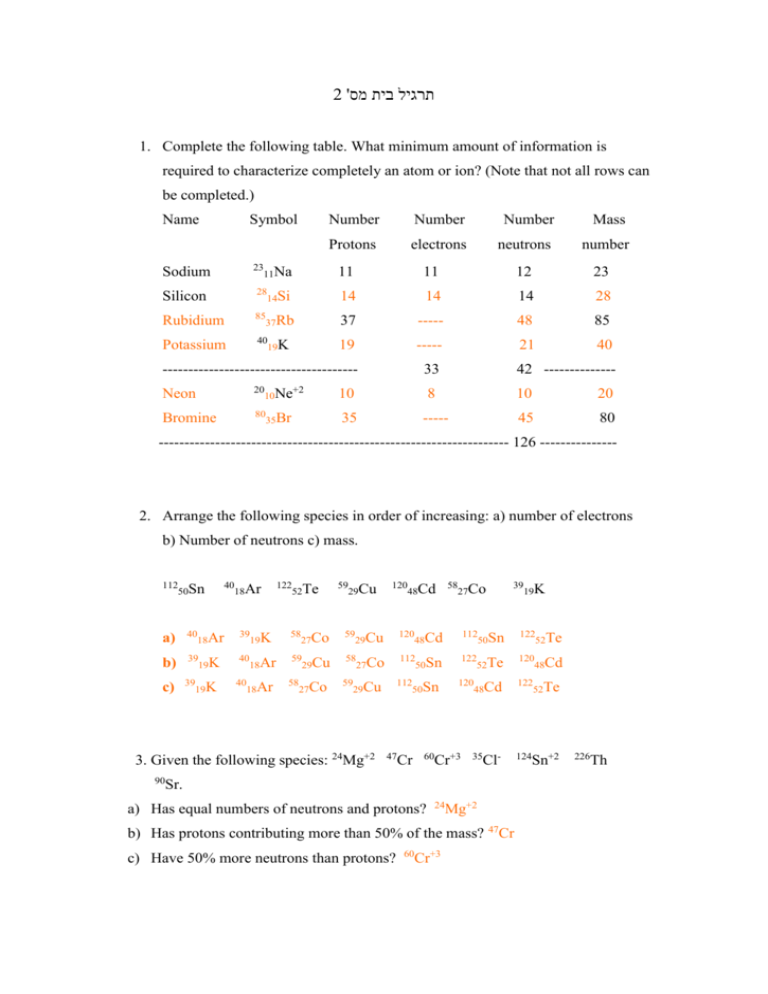
2 'תרגיל בית מס 1. Complete the following table. What minimum amount of information is required to characterize completely an atom or ion? (Note that not all rows can be completed.) Name Symbol Number Number Number Mass Protons electrons neutrons number Sodium 23 11Na 11 11 12 23 Silicon 28 14Si 14 14 14 28 Rubidium 85 37Rb 37 ----- 48 85 Potassium 40 19K 19 ----- 21 40 -------------------------------------- 33 42 -------------- Neon 20 10 8 10 20 Bromine 80 35 ----- 45 80 +2 10Ne 35Br -------------------------------------------------------------------- 126 --------------- 2. Arrange the following species in order of increasing: a) number of electrons b) Number of neutrons c) mass. 112 50Sn 40 18Ar 122 52Te 59 29Cu a) 40 18Ar 39 19K 58 27Co 59 b) 39 19K 40 59 29Cu 58 c) 39 19K 40 18Ar 58 18Ar 27Co 120 48Cd 58 27Co 39 19K 120 48Cd 112 50Sn 122 52Te 27Co 112 50Sn 122 52Te 120 48Cd 59 29Cu 112 50Sn 120 48Cd 122 29Cu 3. Given the following species: 24Mg+2 47 Cr 60 Cr+3 35 Cl- 90 Sr. a) Has equal numbers of neutrons and protons? 24 Mg+2 b) Has protons contributing more than 50% of the mass? 47Cr c) Have 50% more neutrons than protons? 60 Cr+3 124 52Te Sn+2 226 Th 4. What is the total number of atoms in each of the following samples? a) 12.7mol Ca. b) 0.00361mol Ne. c) 1.8X10-12 mol Pu a) 1 mol Ca 12.7 mol Ca 6.023*1023 atoms X atoms 12.7*6.023*1023 = 7.64*1024 mol 1 The total number of atoms in 12.7 mol Ca is: 7.64*1024 b) 1 mol Ne 0.00361 mol Ne 6.023*1023 atoms X atoms 0.00361*6.023*1023 = 2.17*1021 mol 1 The total number of atoms in 0.00361 mol Ne is: 2.17*1021 c) 1 mol Pu 1.8*10-12 mol Pu 6.023*1023 atoms X atoms 1.8*10-12*6.023*1023 = 1.08*1012 mol 1 The total number of atoms in 1.8*10-12 mol Pu is: 1.08*1012 5. Calculate the quantities indicated. a) The number of moles represented by 2.18X1026 Fe atoms. b) The mass in grams of 7.71 mol Kr. c) The mass in mg of a sample containing 6.15X1019 Au atoms. a) 1 mol Fe X mol Fe 6.023*1023 atoms 2.18*1026 atoms 1*2.18*1026 =361.94 atoms 6.023*1023 The number of moles represented by 2.18X1026 Fe atoms is: 361.94 moles. b) 1 mol Kr 7.71 mol Kr 83.80 gr X gr 7.71*83.80 = 646.1 gr 1 The mass of 7.71 mol Kr is: 646.1 gr. c) 6.023*1023 atoms Au 6.15*1019 atoms Au 196.97 gr X gr 6.15*1019*196.97 = 0.02 gr 6.023*1023 0.02*1000=20.11 The mass of a sample containing 6.15X1019 Au atoms is: 20.11 mg. 6. Hydrogen and chlorine atoms form simple diatomic molecules with H and Cl in a 1:1 ratio that is HCl. The natural abundances of the chlorine isotopes are 75.53% 35 Cl and 24.47% 37Cl. The natural abundances of 2H and 3H are 0.015% and less than 0.001% respectively. How many different HCl molecules are possible and what are their mass numbers (i.e. the sum of the mass numbers of the H and Cl atoms)? 35 Cl + 1H = 36 HCl 35 37 HCl 35 38 HCl 37 38 HCl 37 39 HCl 37 40 HCl Cl + 2H = Cl + 3H = Cl + 1H = Cl + 2H = Cl + 3H =







