here - A-level chemistry
advertisement

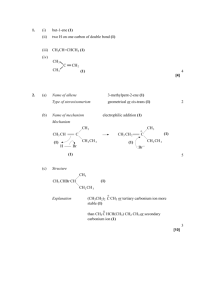
EXTRA ALIPHATIC ORGANIC QUESTIONS – MARK SCHEME SECTION A – MULTIPLE CHOICE 1. D 2. C 3. B 4. B 5. B 6. D 7. C 8. D 9. B 10. A 11. D 12. B 13. B 14. B SECTION B – SHORT ANSWERS 15. (a) (i) Initiation (1) Cl2 2Cl (1) Propagation (1) e.g CH4 + Cl CH3 + HCl (1) Termination (1) e.g CH3 + Cl CH3Cl (1) (ii) (b) to minimize further substitution (1) nucleophilic substitution (1) (1) CH 3 CH 2 Br CH 3 CH 2 OH (1) (1) H – (c) sodium salt of long chain carboxylic acid (1) CH 2 CH CH 2 (1) OH OH OH 2 [13] Mill Hill High School 1 16. (a) (i) methyl ethanoate (1) (ii) CH3COOCH3 + H2O CH3COOH + CH3OH (1) (iii) Catalyst (1) 3 (b) solvents/plasticizers (1) (c) (i) (ii) Line with the highest m/z (1) 1 O R C OC4 H 9 101 R = 116 – 101 = 15 CH3COOC4H9 (1) Molecular formula = C6H12O2 (1) 3 [7] 17. (a) (i) O CH 3 CH CH 2 CH 3 + CH 3 COOH CH 3 C CH 3 O OH + H2O CH CH 2 CH 3 eqn (1) (1) (ii) ester (1) solvent, flavourings (1) (iii) conc H2SO4 (1) in same physical state (1) 6 (b) C4H10O + 6O2 4CO2 + 5H2O (1) 1 (c) (i) CH 3 CH CH 2 CH 3 CH 3 OH (1) CH CH 2 CH 3 CH 2 H (1) + OH 2 + CH CH 2 CH 3 (1) (1) H+ CH 2 = CHCH 2 CH 3 (ii) two H on carbon in double bond (1) (iii) CH 3 CH 3 C H (1) or C H cis but-2-ene (1) H CH 3 C H C CH 3 trans but-2-ene 7 [14] Mill Hill High School 2 18. (a) (i) ethyl ethanoate H H O C C O H (b) H H C C H H H (1) 2 (ii) esterification / condensation / addition - elimination (1) 1 (i) aqueous / dilute sulphuric / hydrochloric acid (allow HCl(aq); H2SO4(aq) not water) (1) temp. < 100° / warm / heat / reflux (this mark dependent on sensible reagent) (1) 2 (ii) CH3COOC2H5 + H2O CH3COOH + C2H5OH (allow C4H8O2, C2H4O2 but must have C2H5OH) (1) (c) 1 sodium hydroxide / sodium carbonate / sodium hydrogen carbonate (allow formula) (1) room temperature / aqueous (2nd mark dependent on correct reagent) (1) (d) 2 ethanamide not ethylamide. H H O C C N H H H (1) 2 [10] 19. (a) (allow O CH 3 CH 2 C COO ) (1) 1 (1) OCH 2 CH 3 (b) Name of mechanism: (nucleophilic) addition- elimination (1) O C Mechanism: CH 3 CH 2 (1) :O CH 3 CH 2 :O (1) Cl H CH 3 CH 2 C Cl O H CH 3 CH 2 structure incl charges (1) 3 arrows (1) 5 [6] Mill Hill High School 3 20. (a) 2-chloropropanoic acid (1) (b) (1) Cl CH 3 CH (1) 1 CH 3 COOH CH COOH + N H ( :NH 3) H CH COOH NH 2 H (1) (1) :NH3 CH 3 (1) Allow SN1 5 (c) (i) H + (Cl – ) H 3 N (CH 2) 4 C COOH +NH 3 (ii) (1) – (Cl ) H H 2N (CH 2) 4 C COO – (Na + ) (1) NH 2 (iii) H H 2N (CONH) O H H C C N or C COOH (CH 2 )4 (CH 2 )4 NH 2 NH 2 H 2N H O H H (CH 2 ) 4 C C N (CH 2 ) 4 C (1) NH 2 COOH NH 2 Or anhydride 3 [9] 21. (a) (b) (c) Lone pair on N (1) accepts a proton (1) 2 (i) Nucleophilic substitution or alkylation (1) (ii) Compound 1 (CH3CH2)2NH (1) Compound 2 (CH3CH2)3N (1) Compound 3 (CH3CH2)4N+Br– Equation CH3CN + 2H2 CH3CH2NH2 (1) Reason only one product formed (1) 4 2 [8] (a) electrophilic addition H 2C (1) CH 2 Cl Cl Mill Hill High School (1) + H 2C (1) (1) – Cl CH 2 (1) Cl : 22. 5 4 (b) CH 2 C 2 H 4 Cl 2 CH 2 H H or C Cl C H + HCl (1) 1 (1) 1 (c) Cl ester or alkoxy alcohol Cl (d) (i) HO–CH2–CH2–OH (1) (ii) high electron density of double bond repels OH– or nucleophile (1) (1) 3 [10] 23. (a) ethanenitrile / ethanonitrile / methyl cyanide / cyanomethane / acetonitrile (1) 1 (b) any hydrolysis (1) 1 (c) CH3COCl + CH3NH2 ® CH3CONHCH3 + HCl for correct formula of methylamine / HCl product (1) overall correct (1) 2 [4] 24. (a) (i) 2, 3 – dimethylbutan – 2 – ol (1) (ii) elimination (1) Mechanism (1) H + (1) OH2 (CH 3 ) 2 C CH (CH3 ) 2 + OH2 (CH 3 ) 2 C CH (CH3 ) 2 (1) H + (CH 3 ) 2 C C (CH 3 ) 2 (CH 3 ) 2 C C (CH 3 ) 2 (1) (iii) Structure CH (CH 3 ) 2 H2 C (1) C CH 3 Name of isomer Explanation Mill Hill High School 2, 3 – dimethylbut – 1 – ene (1) loss of H+ or H (1) from end C also possible (1) 10 5 (b) (i) Equation OH (CH3 ) 2 C OCOCH 3 CH (CH3 ) 2 + CH3 COCl Name of mechanism (CH3 ) 2 C CH (CH3 ) 2 + HCl (1) addition – elimination (1) Mechanism Cl (1) R R O: C H CH 3 + O (1) H (ii) (1) O R Cl (1) + O C O– H CH 3 allow loss of H + here O C CH 3 Type of reaction Reagent(s) Conditions esterification (1) CH3COOH or ethanoic acid (1) strong acid catalyst (1) or H2SO4 or HCL 9 [19] 25. (a) (b) graphical structure for CH3CH2COOH (1) allow CH3CH2– or C2H5– (i) 1 ethanol / CH3CH2OH / C2H5OH not just alcohol (1) (concentrated or dilute) sulphuric acid / HCl / strong acid / H+ not just acid solution heat / reflux / warm / temperature < 100 °C (1) allow second and third marks if alcohol given third mark is dependent on first and second marks second mark is independent on first mark 3 (ii) ethyl propanoate (1) 1 (iii) CH3CH2COOH + CH3CH2OH CH3CH2COOCH2CH3 + H2O C3H6O2 + C2H6O C5H10O2 + H2O (minimum for mark) (1) CH3CH2COOCH2CH3 or C2H5CO2C2H5 (1) (c) (i) compounds with the same molecular formula / same numbers of same atoms (1) but different structural formulae / structures or (1) atoms joined / bonded / linked in different orders / C skeletons not atoms arranged differently (ii) 2 2 sodium ethanoate / CH3COONa (1) propan-1-ol / CH3CH2CH2OH / propanol / propan-2-ol (1) 2 [11] Mill Hill High School 6 26. (a) Name nucleophilic addition (1) Mechanism – (1) O CH 3 C – NC: (b) CH 3 H+ O: C (1) CH 3 CH 3 CN (1) (1) 5 Equation CH3COCH3 + 2[H] CH3CH(OH)CH3 (1) Reducing agent NaBH4 (1) 2 [7] 27. (a) Structure of P: CH 3 (1) CH 2 CH 3 3 C CH CH 3 H CH 2 CH 3 C Structures of Q and R: CH 3 and C CH 2 CH 3 H H C C CH(CH 3 )2 H NOT C 3 H 7 (1) (1) Q and R in any order (b) (i) Racemic mixture: equal mixture of optical isomers / enantiomers OR in explanation Explanation: planar ( >C=O) (1) attack from either side is equally likely (1) (ii) Reagent S: HCN or (KCN / HCl or H2SO4) (1) Compound T: (1) OH CH 3 CH 2 C CH 3 CN Compound U: CH 3 CH 3 (1) C H 6 C CN [9] 28. (a) NaBH4 (1) (b) nucleophilic addition (1) (1) H + – O: (1) O CN – (1) :CN (1) Mill Hill High School 1 (1) 5 7 (c) (i) hexanedioic acid (ii) C6H10O Mr = 98 (1) 2.40 g (1) C6H10O4 Mr = 146 (1) 2.40 × 146 = 3.58 g (1) 98 4 [10] 29. (a) (i) nucleophilic addition (ii) 2-hydroxybutanenitrile H (b) (i) H H H C C C H H H C (1) condone missing hyphen (c) (1) N CH3CH2CH2Br + KCN CH3CH2CH2CN + KBr allow C3H7Br (ii) 1 (1) 2 (1) 1 (1) 1 allow C4H7N nucleophilic substitution / SN2 CN– or NC– (1) lone pair of electrons on C atom (1) 2 [7] 30. (a) (b) (c) (d) Lone pair on N or electron density on N more available or electron density increased electron donation or inductive effect (1) (1) (1) Reagent(s) LiAlH4 or Na/EtOH or H2/Ni (1) Type of reaction reduction or hydrogenation (1) Equation CH3CN + 4[H] or 2H2 CH3CH2NH2 (1) (C2H5)4N+Br– (1) quaternary ammonium salt (1) cationic surfactant or fabric softener (1) Name of mechanism addition-elimination (1) 3 3 3 Mechanism C 2 H 5 NH 2 C (1) (1) O Cl + C 2 H 5 NH 2 CH 3 C O CH 3 + C 2 H 5 NH (1) Mill Hill High School : Cl : (1) H O C CH 3 5 8 (e) C2H5NH2 + (CH3CO)2O C2H5NHCOCH3 + CH3COOH or 2C2H5NH2 + (CH3CO)2O C2H5NHCOCH3 + CH3COO– (1) H C H N 3 2 5 1 [15] 31. (a) (i) H+ or proton acceptor (1) CH3NH2 + H2O ( ) CH3+NH3 (+) OH– (1) (ii) CH3NH3Cl or HCl (1) Or any ammonium compound or strong acid name or formula (iii) + extra OH– reacts with CH 3 NH 3 or reaction / equilibrium moves to left or ratio salt / base remains almost constant (1) Any 2 5 (b) (c) lone pair (on N accepts H+) (1) CH3 increases electron density (on N) donates / pushes electrons has positive inductive effect (1) 2 nucleophilic substitution (1) C2 H 5 CH 3 + N C2 H 5 (1) 2 C2 H 5 [9] 32. (a) (b) 2-amino(e) propanoic acid (1) (i) molecules with same structure / structural formula (1) but with bonds (atoms or groups) arranged differently in space (3D) (1) (ii) (c) Plane polarised light (1) Rotated (equally) in opposite directions (1) (1) H H2N C H 4 O C O– allow H2NCH2COO– Penalise NH2- and OH- once per paper but CH3– is allowed 1 Mill Hill High School 9 (d) CH 3 H2 N C H H (CONH) O C N C C OH O H H H H2 N C H CH 3 C N O C H O C (1) (1) OH H Not anhydrides; not repeating units 2 H (e) H2N C (1) O C H O CH 3 or H2NCH2COOCH3 1 [9] 33. (a) elimination (1) (b) melting point increases (1) boiling point increases(1) or they are liquids, the higher members are solids(1) density increases(1) viscosity increases(1) (c) (d) max 2 addition (1) polymerisation (1) 2 (i) C2H4 + H2O C2H5OH - must show the functional group (1) 1 (ii) vapour phase / high temperature (300 ± 50°C) (1) high pressure 70cl ± 20 (1) if high T and high p, then only 1 mark, value for either gives 2nd mark strong acidic catalyst /H3PO4 (1) (iii) 3 electrophilic (1) addition (1) 2 [11] Mill Hill High School 10 34. (a) (i) (ii) the joining together of monomers / small molecules (1) to form long chains / large molecules (1) nCH2 = CH2 (-CH2–CH2-)n (1) allow n CH2 CH2 not n C2H4 1 (b) 1,2-dibromoethane (1) (c) electrophilic addition (1) H H C C H Br Br (d) + H H H H C C Br :Br – H H H H C C Br Br – (1) correct carbocation intermediate (allow triangular representation) (1) attack by Br– (onto +ve carbon) leading to correct product (1) (i) (1) (iii) 1 H words or diagrams to show attack by p electrons on Br atom and either +/– on Br2 or e– shift on Br–Br (ii) 2 C 38.71/12 = 3.23 ; H 9.68/1 = 9.68 ; O 51.61/16 = 3.23 ratio C:H:O = 1:3:1 /empirical formula = CH3O (1) empirical mass = 31 so molecular formula = 2 × CH3O = C2H6O2 (1) reagent = NaOH / KOH (1) conditions = aqueous solution (dependent on first mark) (1) CH2BrCH2Br + 2NaOH 4 3 2 CH2(OH)CH2OH + 2NaBr product = CH2(OH)CH2OH (condone missing brackets) (1) correctly balanced (1) 2 if C2H6O2 given, allow second mark only for CH2 Br CH2 Br + 2H2O CH2(OH)CH2(OH) + 2HBr allow 2 marks if reagent in (ii) is H2O or aqueous solution [15] 35. (a) (i) O O C 1 C HO OH (ii) H Mill Hill High School (1) O H H C C H H allow HOCH2CH2OH (1) O 1 H 11 O O C (iii) C O O H H C C H H n ester linkage correct ie –COO–CH2– shown as fully graphical structure (1) rest of molecule correct including n (1) repeat unit may start and finish in different place allow e.c.f. from (a)(ii) 2 (b) polyesters (1) (c) addition: joining together (of monomers with double bond) one product only (1) (d) 1 condensation: also involves the elimination of a small molecule (1) allow specific example e.g. H2O, HCl, CH3OH 2 poly(ethene) / poly(propene) condone missing brackets (1) 1 [8] (a) add Fehling’s, warm, should see orange precipitate or add Tollen’s, heat, should see silver mirror (3) propenoic acid (1) (b) 36. H H C H C H H CHO H + Br Br C C H H H C C + H CHO Br Br : CHO Br (4) electrophilic addition (1) [9] SECTION C – LONG ANSWERS 37. (a) 3 Ketones: CH 3 CH 3 CH 2 CH 2 C CH 3 (1) O CH 3 CH C O CH 3 (1) CH 3 CH 2 C CH 2 CH 3 (1) O 3 (b) 4 aldehydes: Mill Hill High School 12 CH 3 H CH 3 CH 2 CH 2 CH 2 (1) C CH 3 O H CH CH 2 (1) C O CH 3 H CH CH 3 CH 2 H (1) C CH 3 O CH 3 Y (1) (c) O CH 3 Z (1) nucleophilic (1) C C X (1) 5 addition (1) equal (1) mixture of optical isomers (1) CH 3 CH 2 e.g 4 (1) C CH 3 CH 2 (d) OH CN Reagents are oxidizing agents (1) Aldehydes can be (easily) oxidized (1) Ketones are not (easily) oxidized (1) 3 [15] 38. nucleophile = electron pair donor or electron rich species which seeks electron deficient site (1) 1 With C2H5Br : reagent : KCN (1) product : propanenitrile (1) mechanism : nucleophilic substitution (1) (1) CH 3 (1) CH 2 – CH 3 CH 2 CN + :Br Br (1) – :CN 6 With CH3CH2CHO reagent : HCN (1) (or KCN/H+) product : 2–hydroxybutanenitrile (1) mechanism : nucleophilic addition (1) H CH 3 CH 2 (1) (1) C – :CN H CH 3 CH 2 O (1) C CN – O: H+ (1) O CH 3 C (1) O CH 2 CH 2 CH 3 8 [15] 39. 3-bromo, 3-methylbut-1-ene (1) the mechanism proceeds via a tertiary carbocation (1) Mill Hill High School 13 other products are formed via primary or secondary carbocations (1) which are less stable so less abundant (1) 4 [4] 40. (a) fractional or primary distillation not just distillation (1) crude oil is useless (1) or products are more useful / valuable than crude oil (b) 2 heat / high temperature (400 °C – 600 °C) (1) catalyst / steam / high pressure / named catalyst known to work eg Al2O3 (1) C4H10 C2H4 + C2H6 or C4H10 2C2H4 + H2 (1) (c) 3 2C2H4 + O2 2CH3CHO (1) 2CH3CHO + O2 2CH3COOH (1) not [O]; not molecular formulae (d) CH 3 C O (e) CH 3 (1) C O 2 1 O ethene < ethanal < ethanol < ethanoic acid (2) give 1 mark for ethene < ethanal < ethanol give 1 mark for ethanol < ethanoic acid ethene has van der Waals’ forces / weak intermolecular forces (1) ethanal has (permanent) dipole-dipole attractions not just ‘ethanal is polar’ (1) ethanol has hydrogen bonding (1) ethanoic acid has more or stronger hydrogen bonding / forms dimers (1) mark reasons independently of order 6 [14] 41. (a) A H H H H C C C H H H H C H H B C O H H C C C C H O H H H (1) H CH 3 C C H H H D H (1) H C C H O O H C O (1) H H C C H H (1) H A = butanal B = methylpropanal Mill Hill High School 14 C = butanone D = ethyl ethanoate (1) Ignore numbers in names unless they make them incorrect spellings must be correct accept alternative trivial names correctly spelled (b) 8 ethanol / correct formula (1) ethanoic acid / ethanoyl chloride / ethanoic anhydride / correct formula (1) temperature less than 100 °C / reflux heat / concentrated sulphuric acid (1) dilute sulphuric acid / acid conditions / H+ (this mark dependent on sensible answers for first two marks) for ethanoyl chloride, room temperature / dry / anhydrous for ethanoic anhydride, heat / up to 100 °C H H H O (c) H C C C H H H 3 or C O H H H CH 3 C C H H O C O H (1) (1) butanoic acid (d) methylpropanoic acid (1) 2 heat with Fehling’s solution / ammoniacal silver nitrate / Tollen’s reagent / other suitable oxidising system eg acidified dichromate / Schiff’s reagent B gives red, green or brown (precipitate) / silver (mirror) or black/grey (1) precipitate / other, dependent on reagent C shows no change (1) B and C can be referred to as ‘aldehyde’ and ‘ketone’ only if names correct in (a) or if there is some other valid identification (e) 3 nucleophilic addition (1) – CH 3 CH 3 C O C – CH 3 CH 2 (1) O CN CH 3 CH 2 H CN (1) for intermediate allow –ve charge on N but curly arrow must come from C CH 3 + OH C CH 3 CH 2 CN (1) for product allow H from HCN or H2O 5 [21] 42. (a) 1, 4-diaminobutane or butane -1, 4-diamine (1) A: BrCH2CH2Br or ClCH2CH2Cl (1) B: NC CH2CH2CN Step 1: Br2 or Cl2 (1) (ignore aq) Step 2: KCN (1) (NOT HCN) Step 3: H2 / Ni or LiAlH4 or Na / C2H5OH (1) (NOT NaBH4) Hydrogenation only for H2 / Ni, or nucleophilic addition only for LiAlH4(1) OR reduction or addition Mill Hill High School 15 7 (1) (b) N (CH 2 ) 4 N C (CH 2 )4 C H H O O (1) QL hydrogen bonding (1) Polarity of H-bonding shown or discussed (1) (c) 4 Polyamides / peptide link can be hydrolysed (1) OR polyalkenes cannot be hydrolysed QL OH– attacks peptide link or C+ (1) poly(ethene) non-polar (1) 3 [14] 43. COOH COOH C C H2N CH3 NH2 CH3 H H (2) asymmetric/ chiral carbon atom (1) 3 [3] 44. (a) combination of several unsaturated molecules (1) to form a single saturated molecule (1) (b) (i) 2 G: hexan-1,6-diol (or structure) (1) H: 1,6-dichlorohexane (or structure) (1) J: hexan-1,6-dioyl dichloride (or structure) (1) K: hexan-1,6-diamine (or structure) (1) Reaction 3: nucleophilic substitution (1) Reaction 5: nucleophilic addition-elimination (1) 6 (ii) O O C (CH2)4 C N H (CH2)6 N H n 2 Mill Hill High School 16 (iii) O + R C Cl H N R Mill Hill High School H - OO R C Cl R R O R C + N + N H Cl + HCl C N H H R H H R 5 [15] 17