THE INTERNAL STRUCTURE OF THE NUCLEUS
advertisement

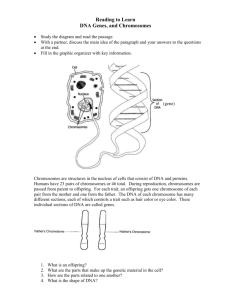
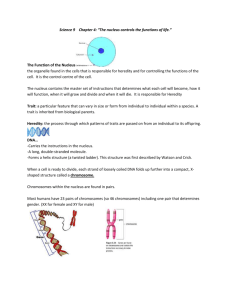
1 THE INTERNAL STRUCTURE OF THE NUCLEUS (1) Textbook: pp. 83-84; pp. 533-536; pp. 539-546. (you need to know what we cover in lecture, These pages are well worth reading. A consideration of the many complex, and simultaneously occurring, reactions in the nucleus suggest there must be compartmentation of reactions in the nucleus. The nucleolus, where the ribosomal subunits are synthesized, is one example of such a compartment but it is becoming increasingly obvious that there are other compartments where specific reactions. occur. None of the nuclear compartments are surrounded by membranes, so they certainly are not membrane-bound organelles! But I, and others, call them organelles anyway, we also do for a chromosome. The integrity of these non-membrane bound organelles is due to the binding of proteins to each other and, probably in all cases, also to DNA and/or RNA. For example, although the nucleolus is not surrounded by a membrane it can be isolated intact from cells. Florescence microscopy has been essential for the recent investigations of nuclear structure and function, involving the use of fluorescent antibodies (immunofluorescence), insertion of genes for GFP fusion proteins, and a technique we have not yet described called fluorescence in situ hybridization (FISH). The latter method allows one to “light up” (by fluorescent tags) DNA sequences instead of proteins. Let us start with a diagram from Biology 111 to refresh our basic knowledge of nuclear structure at interphase: NUCLEAR PORE OUTER NUCLEAR MEMBRANE INNER NUCLEAR MEMBRANE NUCLEAR ENVELOPE NUCLEAR LAMINAR NUCLEOLUS CHROMOSOME EUCHROMATIN (CHROMOSOME IS THE LEAST COMPACTED HER. GENES CAN BE USED) RIBOSOME NUCLEAR PORE CONTAINING NUCLEAR PORE COMPLEX HETEROCHROMATIN (CHROMOSOME VERY COMPACTED HERE) ROUGH ENDOPLASMIC RETICULUM 2 The nuclear lamina is made of intermediate filaments. Amongst its functions are: (1)Maintenance of nuclear shape. (2)Binding of the chromosomes. (3)Help to anchor the nuclear pore complexes (NPCs) The nucleolus is the organelle responsible for: (1)Synthesis of the large and small ribosomal subunits. (2)Synthesis of small nuclear ribonucleoprotein particles (SNURPS). A cell can have more than nucleolus. Human somatic (body) cells right after cell division have eight small nucleus, which in most cell types aggregate to from one large nucleolus. Various amphbians produce oocytes, that have to make very large numbers of ribosomes. These oocytes have a hundred or so nucleoli. How this comes about will become clear later. Diseases involving the mutation of the lamins, a type of intermediate filament protein specific to the nucleus, show that the nuclear lamina is essential for a functional nucleus. The most peculiar disease involving the nuclear lamina is called Hutchinson-Gifford progeria (pro = before, or eary; ger refers to aging, as in geriatrics). In this disease children of ten years old look like they are seniors. They die of strokes and heart disease in their early teens. The nuclei in the cells of these children are mis-shapen. The result of the incorrect shape of these nuclei is that the internal organization of the nucleus becomes non-functional. A lot of this non-functionality is due to incorrect activation and inactivation of genes. We will see below that chromosomes must be properly organized in the interphase nucleus and changes in nuclear shape change this organization. The location of chromosomes in the interphase nucleus We are all aware of what chromosomes look like during mitosis and meoisis. At those times the chromatin is very tighly condensed and all the genes are completely inactivated. The chromosomes have been modified for transport! But what do chromosomes look like at interphase when genes are being used? And what is the is the location of individual cells in the interpahse nucleus? Once again it is fluorescence microscopy that has come to the rescue! It is now possible to “paint” (that is the term used) the individual chromosomes with fluorescence probes (i.e. fluorophores). The technique is called fluorescence in situ hybidization (FISH).The relevant DNA probes can now be bought from molecular biology companies. Preparing the DNA probes for FISH: (1) Much of the DNA of higher organisms does not occur in genes, but instead is located between the genes. This DNA is called highly repetitive DNA. (2) Fortunately for “chromosome painting”, each pair of homologous chromosomes has some highly repetitive DNA sequences that are specific for that pair. And they are located all along the chromosomes. (3) Short segments of these specific DNA sequences can be isolated and copies can be made. (4) The organic molecule biotin can then be covalently bound to various nucleotides of these copied DNA sequences. The nucleotides are said to be biotinylated. 3 Use of the biotinylated DNA probes during FISH: (1) Fix the cells e.g. with formaldehyde or glutaraldehyde. (2) Heat the slide with hot (60C) salt solution. Under these conditions a lot of the DNA in the chromosomes “melts” i.e. the H-bonding between the strands can no longer hold the strands together and they separate. (3) The biotinylated probes are then added. These short DNA strands can then approach their complementary sequences in the chromosomes. (4) The slide is then cooled to room temperature. Because so much of the biotinylated segments are added, there is a good change when the DNA double helices are re-formed (re-annealed). (5) Add fluorescently labelled avidin. Avidin is a protein (there is a lot of it in egg whites) that binds “avidly” to the vitamin called biotin. (6) Observe in fluorescence microscope. If more than one colored “paint” is used then different excitation lights will have to be used and ultimately a composite digital image will be produced. Steps in FISH (diagrammatic) (1) Get a sample of repeating sequence DNA fro a given pair of homologous chromosomes. A T G A A T (2) Make many copies of above sequence by polymerase chain reaction. (3) Covalently attach biotin (bioitinylation) to nucleotides. BIOTIN COVALENT BOND A T G A A T 4 (4) Separate DNA strands of chromosomes in the cells by adding 60C salt solution. A T G A A T T A C T T A The repeated sequence as it occurs in the DNA double helix of the chromosome. Add hot salt solution to the cells on the microscope slide. A T G A A T Strands of the DNA double helix separate as the H-bonds are broken by hot salt solution. T A C T A T (5) Add the biotinylated DNA probe (in high concentration) to the cells on the microscope slide. Let the slide cool. A T G A A T One separated strand of chromosome double helix The added biotinylated DNA probe. H-bonds form between A complementary base pairs at the lower temperature in the absence of the salt T solution T G A A T A C T T A The other separated strand (complementary) of the chromosome double helix 5 (6) Covalently attach fluorophore molecules to the protein avidin. FLUOROPHORE FLUOROPHORE COVALENTLY ATTACHED TO AVIDIN AVIDIN AVIDIN (7) Add fluorophore/avidin complexes to the slide. The avidin will bind to the biotinylated DNA segment. FLUORESCENCE A T G A A T EXCITATION A FLUOROPHORE COAVLENTLY ATTACHED TO AVIDIN AVIDIN BIOTIN A T G A A T T A C T T A DEOXYRIBONUCLEOTIDE Different coloured fluorophores can be attached to the avidin. So what doe we see when each homologous pair of chromosomes is “painted” with FISH? Amazing!! This image shows what can be seen when chromosomes at mitotic metaphase are painted. 6 But is the result that is obtained when the chromosomes at interphase are “painted” that is so spectacular and informative. It is seen that each of the chromosomes has its own “territory” in the interphase nucleus. The term chromatin territorities (CTs) is used. The location of these territories in the interpahse nucleus is sufficiently stable that phylogenteic patterns can be see. There are, however some changes in shape of territories as different genes on the chromosomes are used. These changes seem to allow the segments of different chromosomes to interact depending upon which genes need to be used. Hence there are a variable fringe or overlap regions between individual chromosomes. There are two major ways of regulating gene activity in eukaryotes: (1) Chromatin re-modelling: At the most large-scale level this involves changes in chromosome shape so that given genes are moved into areas where heterochromatin is formed (called gene silencing) or into regions of the nucleus where the more of less condensed chromatin (euchromatin) is favoured. (2) Promoter activation: This involves the binding of transcription factors to the promoter region so that RNA polymerase can bind. (Even this phenomenon actually involves chromatin re-modelling, but at a lesser scale than above.) In bacteria activation of the promoter often involves the removal of a repressor protein from the promoter. Whether it is a case of transcription factors binding to the promoter an activating it (i.e allowing RNA polymerase to bind to the promoter) or a repressor protein bound to the promoter and preventing RNA polymerase form binding, the general idea is the same. That is regulation of gene transcription at the promoter level rather than at the higher structural level described in under “chromatin re-modelling” above. 7 HETEROCHROMATIN EUCHROMATIN Diagram is taken from Alberts et al. (2008) Molecular Biology of the Cell. .If one reflects back upon the disease caused progeria, one can see why inability to maintain a certain nuclear shape could influence to turn genes “on and off” in the correct sequence. It may be that the putting of genes involved in aging into transcription zones may be facilitated by certain nuclear shapes (and thus probably the arrangement chromosome territories?). Or perhaps the genes for protection against aging are more readily put into the “gene silencing” heterochromatin regions with certain improper nuclear shapes. 8