Ayurvedic Herbal Industry: QUEST for Global Acceptance
advertisement

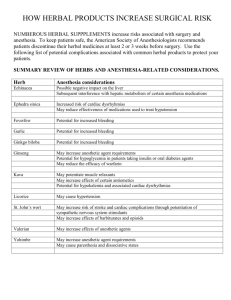
Ayurvedic Herbal Industry: QUEST for Global Acceptance Dr Arun Bhatt MD (Med) FICP (Ind) MFPM (UK) President ClinInvent Research Pvt Ltd Mumbai arunbhatt@clininvent.com Introduction Worldwide, alternative medicine is becoming popular and herbal medicine has become one of the most common forms of alternative therapy. The international herbal market is approximately $61 billion. Annual sales of herbal medicinal products (HMPs) are approximately $3 billion in Germany and $1.5 billion in the US. 1 Annual turnover of Indian Ayurvedic industry is $ 0.8 billion (Rs 35,000 million). 2 The Indian market is growing at 15-20% per annum (Rs 7,000 million or $150 million). With world demand growing at 1% annually ($ 610 million), the size of export market for medicinal plants appears bigger than the Indian domestic market. As compared to China, which boasts of herbal exports of $ 3 billion, Indian exports are dismal - $ 100 million. For Indian herbal industry, there is a huge export opportunity. However, this QUEST is full of challenges of meeting global requirements of Quality, Efficacy, Safety and Standardization. Challenges As the global market is big and expanding, the Indian herbal industry is focusing on exports. From a global perspective, critical challenges for herbal industry are: Regulatory concerns Consumer perceptions Competition Regulatory concerns The regulatory agencies, world over, are focusing on: Quality Efficacy Safety Standardization of herbal medicines. The new guidelines from US FDA and EMEA cover the need for documentation in the above areas.3 Quality – A Major Concern Quality of herbs has become a major concern following reports of heavy metals in Indian herbs.4-6 Adulteration of plants is a serious problem. Some of the common adulterants are: botanicals, toxic metals, microorganisms, microbial toxins, pesticides, and fumigation agents. One study showed that 64% of HMP samples collected in India contained significant amounts of lead (64% mercury, 41% arsenic and 9% cadmium). 5 A recent Harvard Medical School study reported that 14 (20%) of 70 HMPs contained heavy metals.6 However, this problem is not unique to Ayurvedic medicine. Other traditional medicines – Chinese, Middle East and South Americanhave also been implicated. 6 Such contamination can lead to serious harm to patients taking such remedies and could also interfere with the assessment of safety in a clinical trial. Quality has to be assured at all stages – herbal raw materials, processing of herbals and finished herbal medicines. 1 Substantiation of Clinical Efficacy One of the major issues with HMPs is lack of good quality clinical trials. Even if the animal studies or anecdotal clinical experiences are promising and use of an herb is widespread, such observations cannot predict the results of well-designed randomized, controlled trials. Some of the Indian medicinal plants - Phyllanthus amarus, Picrorhiza kurroa, Tinospora cordifolia, Commiphora mukul, Mucuna pruriens, Boswellia serrata, - have been tested in clinical trials. However, a recent review concluded that evidence-based studies on the efficacy and safety of traditional Indian medicines are limited. 7 As there are few good quality clinical trials on Indian HMPs, international researchers have made efforts to confirm Indian data. In a recent double blind randomized placebo controlled trial, 8-week treatment with guggulipid 1 gm or 2 gm did not improve levels of serum cholesterol in population of adults with hypercholesterolemia, and raised levels of LDL-C. 8 Besides, 6 participants taking gugulipid developed a hypersensitivity rash. Such studies cast doubt on the quality of clinical trials of HMPs conducted in India. Most regulatory authorities ask documentation on clinical efficacy of HMPs. Department of AYUSH recommends that manufacturers would be expected to conduct efficacy and safety studies before licenses are granted for Ayurvedic Patent and Proprietary medicines.2 Safety Issues - Adverse Reactions and Drug Interactions Herbal medicines are generally considered comparably safer than synthetic drugs. However, recent reports challenge such assumptions. 9 Ephedra marketed as a dietary aid in USA, led to at least a dozen deaths, heart attacks and strokes. Other well-known safety issues have been hepatotoxicity of kava and renal effects of aristolochic acid. Besides, drug interactions of herbal drugs are of a serious concern. Serious adverse effects have been reported when the addition of St. John's wort caused serum levels of cyclosporine and antiretroviral agents to fall to sub therapeutic levels. Garlic is reported to increase clotting time in patients taking warfarin. WHO has urged the governments to establish regulatory mechanisms to control the safety and quality of products. 10 Standardization of herbal drugs For safe and effective use of herbal drugs, consistency in composition and biologic activity are essential. However, herbal drugs frequently fail to meet this standard, because there are problems of 1) difficulties in identification of plants, 2) genetic variability, 3) variations in growing conditions, 4) diversity in harvesting procedures and processing of extracts, and 5) the lack of information about active pharmacologic principles. 11 In a study of ginseng preparations, the amount of ginsenosides varied from 11.9-327.7% of the amount on the label 12 Medical letter cautions, “Their (herbal medicines) potency may vary and their purity is suspect,” 13Australian medicines regulatory body the Therapeutic Goods Administration, recalled over 1500 HMPs and suspended production license of Pan Pharmaceuticals after an audit, which revealed problems with company's quality control standards. 14 The Indian companies must focus attention on quality during the whole process chain from accessing raw materials to finished products – to meet global expectations. Consumer Perceptions HMPs have become popular because of perceived safety and economy and inability of allopathy to cure everything. However, recent reports of contamination and potential for adverse reactions, 2 have tempered the enthusiasm of consumers for these "natural" cures, resulting in decline of sales of herbal products in the United States.12 The consumers now want more authentic information on quality, safety and efficacy of HMPs. The medical perceptions about complementary medicine (including HMPs) are diverse. 15, 16 Some surveys show that, overall, physicians believe it is moderately effective, while many doctors regard complementary therapies as scientifically unproved. The doctors' are concerned about 1) use of such therapies as an adjunct or an alternative to conventional care, 2) comparative efficacy of complimentary and allopathic therapies and 3) the possibility of adverse effects. In general, globally, the trend amongst doctors is to support the patients' preferences for complementary therapies. However, they want published information from reliable sources on quality, safety and efficacy of HMPs. Competition Amongst the countries with herbal resources, China is a major competition. The discovery of artemisinins as a new class of anti-malarial drugs from Chinese plant Artemisia, has brought Traditional Chinese Medicine (TCM) practices and Chinese HMPs made attractive for research. A random search of MedLine showed that number of publications on TCM was >3 times the number of publications on Ayurveda. The indications for clinical trials of TCM cover the current medical challenges of Allopathy. Some of the therapeutic areas of TCM clinical trials are: neurology, oncology, cardiology, diabetic complications, rheumatoid arthritis etc. These clinical trials are conducted according to scientific and ethical principles of modern clinical research. Chinese government highly values the development of TCM and has announced that by 2010 it will establish a modern TCM innovation system along with a series of standards and norms for modern TCM products, support development of a number of new TCM products and key technologies, and encourage creation of a competitive modern TCM industry. The government efforts are intended to boost the quality of Chinese medicine and enhance China's ability to compete in world markets. Conclusions The future of Indian herbal industry depends on how it prepares it self to face the challenges of the present – regulatory concerns, consumer perceptions, and competition. The global regulatory agencies – US FDA, European Community – have made guidelines for botanicals. 2 Recently, The Australian government has backed increased regulation of the complementary health sector. These guidelines focus on documentation of the key issues Quality, Efficacy, Safety, and Standardization. Some of these issues will also be applicable to dietary supplements. The international regulatory authorities would expect the data generated (pre-clinical, CMC and clinical) should meet the standards of GxPs (Good Practices) – good agricultural practices, good laboratory practices (GLP), good clinical practices (GCP) and good manufacturing practices (GMP). These guidelines will make licensing difficult for HMPs. Besides, the governments are likely to restrict availability of HMPs with toxic potential. WHO has also recommended that it important for governments 10 to establish regulatory mechanisms to control the safety and quality of products and of TM/CAM practice. The consumers – doctors and patients- expect innovation and effective options for chronic diseases. The industry has to 1) become creative in designing clinical trials, 2) developing consumer friendly products and 3) effective marketing communication. Table 1 and 2 suggest some innovative options for developing consumer friendly medicines. 3 The future belongs to an herbal company that is research-focused, quality-driven, regulatorycompliant, consumer-friendly and market-savvy! References 1 De Smet PAGM Herbal Remedies N Engl J Med 2002 347: 2046-56 2 Department of Indian System of Medicine and Homeopathy Draft National Policy 2001 www.indianmedicine.nic.in 3 Bhatt A D Regulatory Issues for Herbal Medicines Ayurvedline 7th Edition 2004 p 95 -103 4 Ernst E. Heavy metals in traditional Indian remedies Eur J Clin Pharmacol 2002 57:891-6 5 Saper RB, Kales SN, Paquin J et al RS Heavy Metal Content of Ayurvedic Herbal Medicine Products JAMA 2004; 292:2868-2873 6 Ernst E, Thompson Coon J Heavy metals in traditional Chinese medicines: A systematic review Clin Pharm Therap 2001 70 7 Ladha R, Bagga A Traditional Indian system of medicine Ann Acad Med Singapore 2000 29:3741 8 Szapary PO, Wolfe ML, Blaydon L T et al Guggulipid for the Treatment of Hypercholesterolemia A Randomized Controlled Trial JAMA 2003;290:765-772. 9 Stein MC Are herbal products dietary supplements or drugs? An important question for public safety Clin Pharmacol Therap 2002; 71: 411-13 10 WHO Fact Sheet N°134, Revised May 2003 11 Marcus DM, Grollman AP Botanical Medicines — The Need for New Regulations N Engl J Med 2002 347:2073-2076 12 Straus SE Herbal Medicines — What's in the Bottle? N Engl J Med 2002 347:1997-1998 13 Problems with dietary supplements Med Lett Drugs Ther 2002 44:84-6 14 Complementary medicines industry in crisis after recall of 1546 products BMJ 2003 326:1001 15 Zollman C, Vickers A . ABC of complementary medicine Complementary medicine and the doctor BMJ 1999;319:1558-1561 16 Ernst E, Resch KL, White AR. Complementary medicine. What physicians think of it: a metaanalysis. Arch Intern Med 1995 155:2405-8 4 Table 1 Clinically Relevant Evaluation of Advantages of Medicinal plants (CREAM) Holistic therapy for disease and concomitant conditions – o Poly-herbal for Diabetes mellitus to manage - Hyperglycemia, Hyperlipidemia Adjuvant synergistic therapy to improve response to primary therapy o Issues in Tuberculosis treatment - Hepato-toxicity, Immune-deficiency Niche therapy when there are contraindications or cautions against allopathic agents – o Arthritis with associated problems - Acid peptic disease, Edema, Therapy to provide positive side effects o Cough suppressants and constipation Table 2 Development Rationale for Enhancing Advantages of Medicinal plants (D R E A M) Conversion of powder to tablet / capsule / liquid form Reduction in size of tablet or capsule Reduced frequency of dosing Improved solubility providing a liquid alternative for elderly and children Improved palatability Potential for parenteral formulation 5