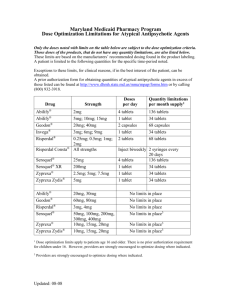
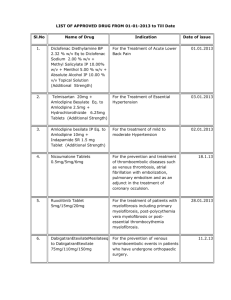
Complying to 91/155/EC, 93/112/EC

Complying to 91/155/EC, 93/112/EC
Product Safety Data Sheet
Benazecare 5mg and 20mg Tablets
1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION
Product Name Benazecare 5, Benazecare 20
Product Code XVD 440, XVD 441
Supplier Animalcare Ltd,
Common Road,
Dunnington,
York, YO19 5RU,
Telephone No. +44 (0) 1904 487687
Fax No. +44 (0) 1904 487611
E-Mail: animalcare@animalcare.co.uk
Emergency Telephone +44 (0) 1904 487687 (daytime)
__________________________________________________________________________
2. HAZARDS IDENTIFICATION
Physical and Chemical Hazards: Unlikely to present a health hazard in normal use
Environmental Hazards: Unlikely to present an environmental hazard in normal use
__________________________________________________________
3. COMPOSITION / INFORMATION ON COMPONENTS
Cas No. FINECS No.
Benazapril Hydrochloride: BG541-74-4
Substance/Preparation: Tablets
Active Ingredients: Benzapril Hydrochloride
Description: Cardiac Therapeutic
Chemical Family: ACE inhibitor
Revision Date: November 2008 Printing Date: 19 December, 2006 Page 1 of 5
Complying to 91/155/EC, 93/112/EC
Product Safety Data Sheet
Benazecare 5mg and 20mg Tablets
4. FIRST AID MEASURES
Inhalation : Remove to fresh air. If anay signs or symptoms occur or persist seek
Medical advice.
Skin Contact : Wash thoroughly with soap and water. Remove contaminated clothing and wash before reuse. Unlikely to arise from broken tablets.
Eye Contact : Immediately flush eyes with copious amounts of water for at least 10 minutes. Immediately obtain medical assistance.
Ingestion : Immediately obtain medical assistance. Subject must be kept warm and at rest.
NOTE TO PHYSICIAN: No empirical data are available for humans. The most significant sign is likely to be marked hypotension. Repeatedly administer medicinal charcoal in a large quantity of water. NOTE; never give anything by mouth to an unconscious person. Do not induce vomiting. The active metabolite, benazeprilat, cannot be eliminated to a significant degree by dialysis. If blood pressure falls severely, administer physiological saline i.v.
5. FIRE FIGHTING MEASURES
Extinguishing Media: The preparation is non-flammable. In the presence of fire, dangerous fumes may be given off .
Extinguishing media
include powder, foam, carbon dioxide or water spray (do
no use direct jet of water). Measures should be taken to
prevent contaminated extinguishing media from seeping
into the ground, contaminating water courses or spreading
uncontrollably.
Protective clothing and breathing apparatus must be worn.
Fire and explosion hazards: None
6. ACCIDENTAL RELEASE MEASURES
Personal Precautions : Wear rubber gloves when handling. Prevent skin and eye contact.
Environmental Precautions: Do not contaminate water courses or sewers.
Revision Date: November 2008 Printing Date: 19 December, 2006 Page 2 of 5
Complying to 91/155/EC, 93/112/EC
Product Safety Data Sheet
Benazecare 5mg and 20mg Tablets
Method for Clean-up : Sweep up and place in a labelles container for safe disposal.
Contact local authority (Environmental Health Department) or
specialist disposal contractor or supplier for information on safe
disposal.
7. HANDLING AND STORAGE
Handling: Avoid contact with eyes, skin or clothing. Avoid inhalation of dust. Wash hands thoroughly after handling.
Storage: Store in closed original containers in a cool, dry, well-ventilated area, below
25
º
C. Store separately from food and feed. Do not re-use containers for any
other purpose. Do not transfer contents to unlabelled containers. Use only with
adequate ventilation. Keep out of reach of children. Unused half tablets should
be returned to the open blister pack, inserted into the cardboard box and
stored safely.
8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Occupational Toxicity Risk: Low (tablet form)
Occupational Exposure Standard: Benezepril Hydrochloride 0.02mg/m з
(USA value)
General Personal Protective Equipment: Unnecessary for the dosage form
Precautionary measures after work: Wash hands if tablets have been broken manually
9. PHYSICAL AND CHEMICAL PROPERTIES
Form: Scored ovoid tablets
Colour: 5mg Tablets – Light Yellow 20mg Tablets – Pink
Odour: None
Revision Date: November 2008 Printing Date: 19 December, 2006 Page 3 of 5
Complying to 91/155/EC, 93/112/EC
Product Safety Data Sheet
Benazecare 5mg and 20mg Tablets
__________________________________________________________
10. STABILITY AND REACTIVITY
Stability: Stable under normal temperatures and pressures. Thermal decomposition occurs above 180 º C. No hazardous decomposition
products when handled according to the instructions.
Conditions to avoid: Sensitivity to humidity – store in dry place
Materials to avoid: None known
11. TOXICOLOGICAL INFORMATION
Acute oral LD50 rat: > 5000mg/Kg (active ingredient)
12. ECOLOGICAL INFORMATION
Fish toxicity (Zebra Fish) LC50 96h >100mg/1
Daphnia Magna (Water flea) >1000mg/1
Toxicity to bacteria (act water sludge, 3h) IC50 > 100mg/1
Environmental hazard in case of accident (spillage/leakage) - slight
13. DISPOSAL CONSIDERATIONS
Product/Residues: Dispose of using a reputable specialist disposal contractor
Package: Dispose of waste containers using regular disposal methods in
accordance with local and national environmental regulations.
14. TRANSPORT INFORMATION
Transport classification: Not classified as hazardous for transportation
Revision Date: November 2008 Printing Date: 19 December, 2006 Page 4 of 5
Complying to 91/155/EC, 93/112/EC
Product Safety Data Sheet
Benazecare 5mg and 20mg Tablets
15. REGULATORY INFORMATION
Registered as: Veterinary Medicine
Market Authorisation Number: 5mg Vm 10347 / 4021
20mg Vm 10347 / 4022
Legal category: POM-V
16. OTHER INFORMATION
Read label thoroughly before use
The data given here is based on current knowledge and experience. The purpose of this safety datasheet is to describe the products in terms of their safety requirements. The data does not signify any warranty with regards to the products properties.
Revision Date: November 2008 Printing Date: 19 December, 2006 Page 5 of 5