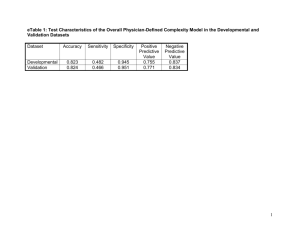
High Efficiency mer-Iridium Complexes for Organic Light Emitting
advertisement

# Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2004 Supporting information Experimental Instrumentation: 1H-NMR and 13C-NMR spectra were measured using a Bruker AMX-400 (400 MHz), EI Mass spectra were collected with a Bruker APEX II, and Photoluminesence spectra were recorded with a HITACHI F-4500. Preparation of green phosphorescence iridium complex, fac-Ir(ppy)3, (ppy)2Ir(acac), mer-Ir(ppy)3 and mer-Ir(m-ppy)3: All procedures involving IrCl3•H2O were carried out in nitrogen gas atmosphere. Cyclometalated Ir(III) μ-chloro-bridged dimers were synthesized by the method reported by Nonoyama13. IrCl3•H2O (Next Chimica) and 2.5 equiv. of 2-phenylpyridine ligands were heated in a 3:1 mixture of 2-ethoxyethanol and water. This slurry was heated at 100℃ for 24 hrs. After cooling to room temperature, the precipitate, [C^N2Ir(μ-Cl)2IrC^N2], was filtered off and washed with water. This dimer was divided into two parts and dry in 60℃. First part, the obtained solid was placed in a flask and dispersed in 2-ethoxyethanol. Acetylacetone and sodium carbonate were added to the solution and the mixture were heated at 120℃ for 12~16 hrs. After cooling to room temperature, the crude product was filtered off and washed with water, followed by 2 portions of n-hexane and ether. The solid was dried in vacuum and zone sublimed to give pure product-(ppy)2Ir(acac). S1 # Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2004 The give pure product-(ppy)2Ir(acac) was placed in a flask and dispersed in glycerol. 2-phenylpridine was added to the solution and the mixture were refluxed for 15 hrs. After cooling to room temperature, the crude product was filtered off and washed with water, followed by 2 portions of n-hexane and ether. The solid was dried in vacuum and zone sublimed to give pure product- fac-Ir(ppy)3. fac-Ir(m-ppy)3 was prepared from the same process. Second part, two different dimer was placed in the train sublimation equipments individually. The dimer was sublimed at 300℃ for 8~12 hrs and get the pure productmer-Ir(ppy)3 and mer-Ir(m-ppy)3. All of these pure products were used for advanced analysis and device fabrication. fac-Ir(ppy)3: iridium (III) fac-tris(2-phenylpyridinato-N,C2’). EIMS: m/z 655, [M]+. 1H-NMR (CD2Cl2, 300MHz): δ 7.92 (d, J = 8.1 Hz, 3H), 7.62-7.68 (m, 6H), 7.56 (d, J = 5.2 Hz, 3H), 6.86-6.95 (m, 6H), 6.73-6.82 (m, 6H). 13C-NMR (CD2Cl2, 75MHz) δ: 156.6, 151.2, 137.3, 134.0, 126.9, 126.4, 119.8, 114.2, 112.3, 110.0, 109.0. fac-Ir(m-ppy)3: iridium (III) fac-tris(2-phenylpyridinato-N,C2’). EIMS: m/z 697, [M]+. 1H-NMR (CD2Cl2, 300MHz): δ 7.73 (s, 3H), 7.64 (d, J = 7.6 Hz, 3H), 7.42 (d, J = 5.3 Hz, 3H), 6.86 (t, J = 7.6 Hz, 3H), 6.71-6.76 (m, 9H), 2.42 (2, 9H). 13C-NMR (CD2Cl2, 75MHz) δ: 156.1, 151.7, 137.9, 136.7, 134.1, 126.9, 119.5, 113.9, 113.3, S2 # Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2004 109.8, 109.7, 11.2. (ppy)2Ir(acac): iridium (III) bis(2-phenylpyridinato-N,C2’)acetylacetonate. EIMS: m/z 600, [M]+. 1H-NMR (CD2Cl2, 300MHz): δ 8.53 (d, J = 5.7 Hz, 2H), 7.91 (d, J = 8.0 Hz, 2H), 7.81 (t, J = 7.3 Hz, 2H), 7.61 (d, J = 7.5 Hz, 2H), 7.22 (t, J = 6.1 Hz, 2H), 6.88 (t, J = 7.3 Hz, 2H), 6.72 (t, J = 7.3 Hz, 2H), 6.27 (d, J = 7.6 Hz, 2H), 5.34 (s, 1H), 1.84 (s, 6H). 13C-NMR (CD2Cl2, 75MHz) δ: 174.9, 158.4, 138.4, 137.6, 135.3, 127.3, 123.3, 118.9, 114.0, 112.0, 111.0, 108.7, 90.5, 18.5. mer-Ir(ppy)3: iridium (III) mer-tris(2-phenylpyridinato-N,C2’). EIMS: m/z 655, [M]+. 1H-NMR (CD2Cl2, 400MHz): δ 8.09 (d, J = 5.6 Hz, 1H), 7.95-7.93 (m, 2H), 7.85 (s, 1H), 7.83 (s, 1H), 7.77 (d, J = 7.7 Hz, 1H), 7.72 (d, J = 7.7 Hz, 1H), 7.68-7.64 (m, 3H), 7.53-7.49 (m, 2H), 6.99-6.76 (m, 10H), 6.59 (d, J = 6.8 Hz, 1H), 6.42 (d, J = 7.6 Hz, 1H). 13C-NMR (CD2Cl2, 100MHz) δ: 167.4, 165.2, 160.6, 158.5, 157.8, 149.6, 143.3, 141.3, 138.0, 135.7, 135.1, 132.6, 127.8, 126.9, 125.9, 124.4, 122.8, 120.6, 120.0, 119.9, 119.6, 114.5, 114.4, 114.1, 112.5, 112.3, 111.6, 111.5, 111.1, 109.3, 109.0, 108.9, 108.6. mer-Ir(m-ppy)3: iridium (III) mer-tris(2-phenyl 4-methylpyridinato-N,C2’). EIMS: m/z 697, [M]+. 1H-NMR (CD2Cl2, 400MHz): δ 9.06 (d, J = 5.9 Hz, 1H), 7.76 (s, 1H), 7.73 (s, 2H), 7.64 (d, J = 7.7 Hz, 2H), 7.53 (d, J = 7.6 Hz, 1H), 7.42 (d, J = 5.7 Hz, 2H), 6.88-6.57 (m, 11H), 5.89 (d, J = 7.7 Hz, 2H), 2.67 (s, 3H), 2.42 (s, 6H). S3 # Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2004 C-NMR (CD2Cl2, 100MHz) δ: 156.1, 151.7, 141.0, 138.4, 137.9, 136.7, 134.3, 13 134.1, 126.9, 120.7, 119.5, 119.0, 113.9, 113.5, 113.3, 111.3, 109.7, 109.6, 109.5, 11.2. OLED Fabrication and Measurement: Pre-patterned ITO glasses with an effective device of 0.16 cm2 were cleaned in detergent for 10min, and then washed with large amount of doubly distilled water. After sonicated in pure water for 5 mins, these glasses were dried in oven at 180℃ for 90 mins. The organic layers were deposited thermally at a rate of 0.1 nm/sec and pressure of ~1x10-6 Torr in a deposition system. Aluminum was deposited as the cathode. The Electrophosphorescence data were measured with a SpectraScan PR650. Electrophosphorescence spectra for the iridium complexes were showed in Fig 3. It exhibits the same tendency with photoluminescence spectra. S4 # Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2004 fac-Ir(ppy)3 (ppy)2-Ir(acac) mer-Ir(ppy)3 mer-Ir(m-ppy)3 fac-Ir(m-ppy)3 Relative Intensity (a.u.) 1.0 0.8 0.6 0.4 0.2 0.0 350 400 450 500 550 600 650 700 750 800 wavelength (nm) Fig. 3 EL spectra of iridium complexes. S5