Using the Classic TA-cloning Kit from Invitrogen to isolate individual
advertisement

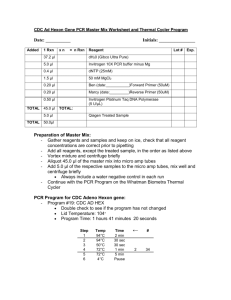
Using the Classic TA-cloning Kit from Invitrogen to isolate individual PCR products from a heterogeneous amplification. You should use this protocol in any the following circumstances: You get a sequence that appears to have a “frame shift”, causing two or more sequences to be overlaid in the chromatogram. You suspect that you have 2 alleles giving you “mixed peaks”. You have difficulty generating enough product from direct PCR. Materials: You will need these items not included in the kit: PCR product. While fresh, clean products are recommended, note that success has been experienced with products cleaned with pellet paint six weeks previously. Adjust the volume to the equivalent of a strong band in 35ul of ddH2O. PCR product density can also be toggled in the ligation reaction. Sterile water Competent E. coli cells (lacking the lac repressor and antibiotic resistance) [people who read this from the web may not understand ha ha] LB plates containing 50ug/ml kanamycin (100ug/ml ampicillin is a suitable substitution) Thermal cycler or other block capable of maintaining 14 oC 42oC heat block or water bath 37oC shaking and non-shaking incubator S.O.C. medium (packets of dry medium will be available for dissolving in sterile ddH2O) X-Gal (always make sure there is plenty left when you are done) Included in the kit (Invitrogen K2020): 10X ligation buffer PCR®2.1 vector T4 DNA Ligase Methods: 1. Set up the following ligation reaction (in 200ul thin wall PCR tube or 1.5 ml eppendorf tube, see below): PCR product Xul (1-3ul is a working range) 10x Ligation Buffer 1ul ® PCR 2.1 vector (25ng/ul) 2ul Sterile water to a total volume of 9ul T4 DNA Ligase (4.0 Weiss units) 1ul Final Volume 10ul 2. Incubate at 14 oC for 4-18hrs in thermal cycler block or other cooling block. (4 hours is a minimum ligation, not necessarily recommended. Overnight is recommended). 3. Place ligation reaction on ice. 4. Place 2 LB-KAN plates per reaction in incubator. 5. Thaw 50ul frozen competent cells on ice (1/2 hour, 1.5ml eppendorf tube if producing your own competent cells …one shot cells are not supplied in the kit we purchase). 6. Bring water bath or heating block to stable 42 oC (This number is crucial. Do not attempt large numbers of reactions in heat block as this will change the temperature for a few moments). 7. Transfer 2ul of ligation reaction to vial of competent cells 8. Incubate on ice for 30 minutes 9. Heat shock cells for exactly 30 seconds at 42 oC, and immediately transfer to ice 10. Add 250 ul of room temperature S.O.C. medium to each vial between 2 and 5 minutes after heat shock. (Do not use S.O.C. medium from another kit, as that kit will then be depleted of necessary supplies.) 11. Shake at 37 oC and 225rpm for 1 hour 12. While shaking, using serile technique spread 50ul of X-gal on LB plates and return them to incubator. 13. Spread 2 different volumes (1 on each plate) of transformed cells on LB-KANXGAL plates (recommend 50ul and 100ul) to insure proper spacing of colonies. 14. Incubate plates overnight at 37 oC White and light blue colonies have been transformed with recombinant plasmids, although they could contain vector sequence, primer inserts or unexpected DNA. 15. Set PCR to screen 10 colonies: a. Use 25ul volume reaction b. Use 10M M13F and M13R primers c. Using 1ul pipette tip, take positive colony, scratch lightly on LB-Amp plate for archiving, then stir and pipette up and down into PCR mixture. d. Run standard PCR protocol in thermal cycler e. Check for products 200bp larger than your product of interest on agarose gel