R3_1
advertisement
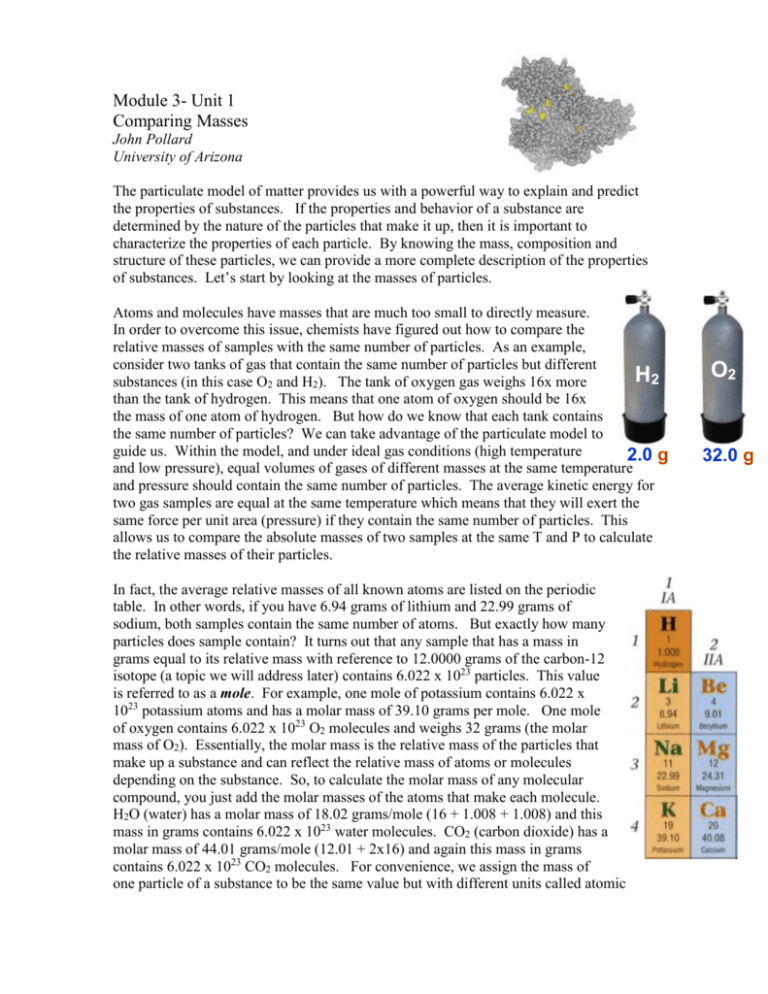
Module 3- Unit 1 Comparing Masses John Pollard University of Arizona The particulate model of matter provides us with a powerful way to explain and predict the properties of substances. If the properties and behavior of a substance are determined by the nature of the particles that make it up, then it is important to characterize the properties of each particle. By knowing the mass, composition and structure of these particles, we can provide a more complete description of the properties of substances. Let’s start by looking at the masses of particles. Atoms and molecules have masses that are much too small to directly measure. In order to overcome this issue, chemists have figured out how to compare the relative masses of samples with the same number of particles. As an example, consider two tanks of gas that contain the same number of particles but different H2 substances (in this case O2 and H2). The tank of oxygen gas weighs 16x more than the tank of hydrogen. This means that one atom of oxygen should be 16x the mass of one atom of hydrogen. But how do we know that each tank contains the same number of particles? We can take advantage of the particulate model to guide us. Within the model, and under ideal gas conditions (high temperature 2.0 g and low pressure), equal volumes of gases of different masses at the same temperature and pressure should contain the same number of particles. The average kinetic energy for two gas samples are equal at the same temperature which means that they will exert the same force per unit area (pressure) if they contain the same number of particles. This allows us to compare the absolute masses of two samples at the same T and P to calculate the relative masses of their particles. In fact, the average relative masses of all known atoms are listed on the periodic table. In other words, if you have 6.94 grams of lithium and 22.99 grams of sodium, both samples contain the same number of atoms. But exactly how many particles does sample contain? It turns out that any sample that has a mass in grams equal to its relative mass with reference to 12.0000 grams of the carbon-12 isotope (a topic we will address later) contains 6.022 x 1023 particles. This value is referred to as a mole. For example, one mole of potassium contains 6.022 x 1023 potassium atoms and has a molar mass of 39.10 grams per mole. One mole of oxygen contains 6.022 x 1023 O2 molecules and weighs 32 grams (the molar mass of O2). Essentially, the molar mass is the relative mass of the particles that make up a substance and can reflect the relative mass of atoms or molecules depending on the substance. So, to calculate the molar mass of any molecular compound, you just add the molar masses of the atoms that make each molecule. H2O (water) has a molar mass of 18.02 grams/mole (16 + 1.008 + 1.008) and this mass in grams contains 6.022 x 1023 water molecules. CO2 (carbon dioxide) has a molar mass of 44.01 grams/mole (12.01 + 2x16) and again this mass in grams contains 6.022 x 1023 CO2 molecules. For convenience, we assign the mass of one particle of a substance to be the same value but with different units called atomic O2 32.0 g mass units. In other words, one mole of CO2 has a mass of 44.01 grams and one molecule of CO2 has a mass of 44.01 amu (atomic mass units). In summary, knowing the relative masses of the particles that make up substance allows us to determine the number of particles in a certain mass or calculate the mass of a certain number of particles. We now have a way of thinking that allows us to think about the mass of individual particles and how this relates to the mass of macroscopic samples of substances. Avogadro’s Number C 12.01 amu Particulate Molar Mass MOLE 6.022 x 1023 units 12.01 g Macroscopic Freon 1) Chlorofluorocarbons, such as CF2Cl2 used to be used as refridgerants in cars and air condition systems but has been banned due to its ability to contribute to the depletion of the ozone layer in our atmosphere. a) Calculate the molar mass of CF2Cl2. b) How many CF2Cl2 molecules are in a 25 gram sample? c) If 25 grams of CF2Cl2 are released into the atmosphere, what mass of chlorine (Cl) are released? d) If a 25 gram sample of CHF2Cl were released, would more or less chlorine be released? Make sure you can justify your answer numerically. e) Despite the fact that chlorofluorocarbons (CFC’s) were banned in 1996, there are still approximately 100 million air conditioning units that still use CFC-12 (CF2Cl2). If each of these air conditioning units contains 1.1 kg of CFC-12 and leak 25% a year, how much chlorine (Cl) is added to the atmosphere yearly?






