The chemistry of porphyrins and related compounds are one
advertisement
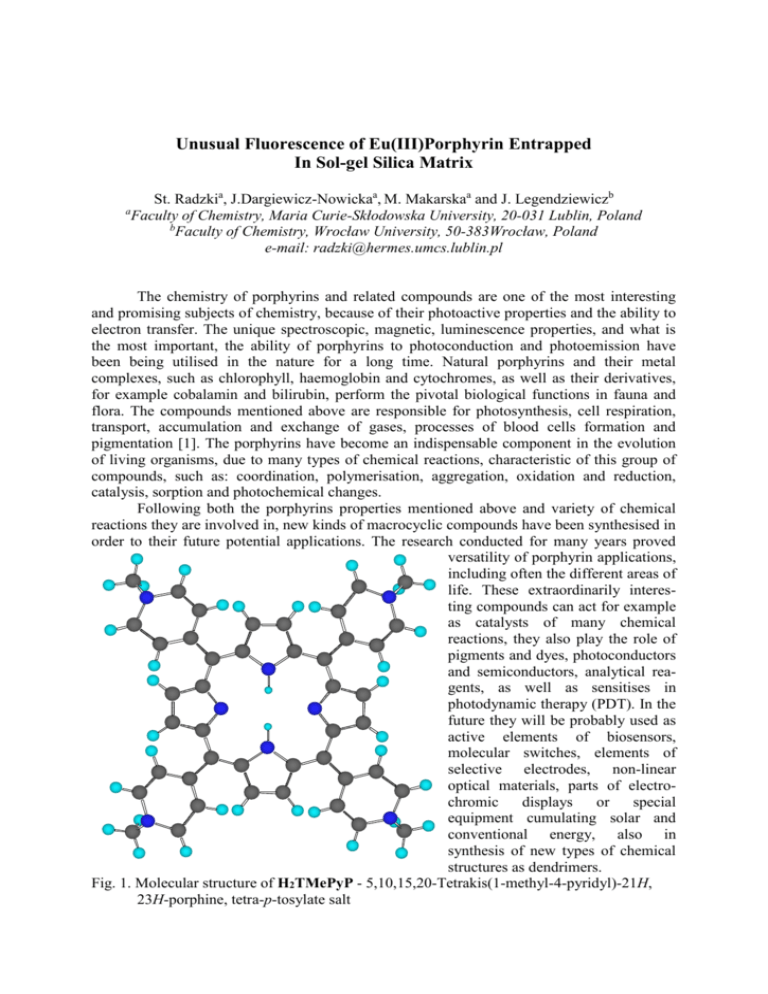
Unusual Fluorescence of Eu(III)Porphyrin Entrapped In Sol-gel Silica Matrix St. Radzkia, J.Dargiewicz-Nowickaa, M. Makarskaa and J. Legendziewiczb a Faculty of Chemistry, Maria Curie-Skłodowska University, 20-031 Lublin, Poland b Faculty of Chemistry, Wrocław University, 50-383Wrocław, Poland e-mail: radzki@hermes.umcs.lublin.pl The chemistry of porphyrins and related compounds are one of the most interesting and promising subjects of chemistry, because of their photoactive properties and the ability to electron transfer. The unique spectroscopic, magnetic, luminescence properties, and what is the most important, the ability of porphyrins to photoconduction and photoemission have been being utilised in the nature for a long time. Natural porphyrins and their metal complexes, such as chlorophyll, haemoglobin and cytochromes, as well as their derivatives, for example cobalamin and bilirubin, perform the pivotal biological functions in fauna and flora. The compounds mentioned above are responsible for photosynthesis, cell respiration, transport, accumulation and exchange of gases, processes of blood cells formation and pigmentation [1]. The porphyrins have become an indispensable component in the evolution of living organisms, due to many types of chemical reactions, characteristic of this group of compounds, such as: coordination, polymerisation, aggregation, oxidation and reduction, catalysis, sorption and photochemical changes. Following both the porphyrins properties mentioned above and variety of chemical reactions they are involved in, new kinds of macrocyclic compounds have been synthesised in order to their future potential applications. The research conducted for many years proved versatility of porphyrin applications, including often the different areas of life. These extraordinarily interesting compounds can act for example as catalysts of many chemical reactions, they also play the role of pigments and dyes, photoconductors and semiconductors, analytical reagents, as well as sensitises in photodynamic therapy (PDT). In the future they will be probably used as active elements of biosensors, molecular switches, elements of selective electrodes, non-linear optical materials, parts of electrochromic displays or special equipment cumulating solar and conventional energy, also in synthesis of new types of chemical structures as dendrimers. Fig. 1. Molecular structure of H2TMePyP - 5,10,15,20-Tetrakis(1-methyl-4-pyridyl)-21H, 23H-porphine, tetra-p-tosylate salt The sol-gel process involves the evolution of inorganic networks through the formation of a colloidal suspension (sol) and gelation of the sol to form a network in a continuous liquid phase (gel). The precursors for synthesising these colloids consist of a metal surrounded by various reactive ligands. Metal alkoxides are most popular because they react readily with water. The most widely used metal alkoxides are the alkoxysilanes, such as tetramethoxysilane (TMOS) and tetraethoxysilane (TEOS). However, other alkoxides such as aluminates, titanates, zirconates or borates may be also used in the sol-gel process, often mixed with TEOS. In sol-gel process the following steps are involved: 1. Hydrolysis of the precursor, catalysed either in acid or in basic conditions Si(OR)4 + nH2O → (OR)4-nSi-(OH)n + nROH 2. Condensation reactions, which may occur with the production of alcohol: (RO)3Si-OR + HO-Si(OR)3 → (RO)3Si-O-Si(OR)3 + ROH or with the production of water: (RO)3Si-OH + HO-Si(OR)3 → (RO)3Si-O-Si(OR)3 + H2O The possibility of mixing organic and inorganic compounds in a new unique hybrid material is realised by sol-gel method. The entrapment of organic reagents into sol-gel matrices and coatings has been the objective of much research since Avnir and co-workers pointed out the role of such systems for sensing purpose [2]. Sol-gel monoliths and sol-gel thin films are very useful to encapsulate various guests such as inorganic clusters, lanthanide complexes, laser dyes and bioactive molecules [3, 4]. The sol-gel immobilisation of porphyrins in suitable matrices has been reported in literature due to the importance of these systems from many points of interest, such as: chemical and biochemical sensing optical limiting, hole-burning and catalysis [5, 6]. Here we present our study on the encapsulation water-soluble free-base H2TMePyP and its complex with Eu(III) in xerogels prepared by the sol-gel method. CH3 CH3 O Eu H3C +N N CH3 N + O N N N CH3 + N N + CH3 Fig. 2. EuTMePyP(acac) Europium(III) acetyloacetonate 5,10,15,20-tetrakis (1-methyl-4-piryfyl) porphine The Eu(III)TMePyP(acac) complex (Fig. 2) was prepared by the method described earlier [7]. The monolith samples with the diameter of about 10 x 10 x 15 mm were prepared by tetraethoxysilane (TEOS) hydrolysis. We investigated their absorption and emission spectroscopic properties compare with the spectra of the same compounds in various solvents. The spectra of europium complex were compared with those of free-base porphyrins. Uv-vis absorption spectra of monolithic gels are shown in Fig. 3. 1 . 1 A S o r e t b a n d 4 2 8 n m Q b a n d ~ 1 5 x 5 2 0 n m 1 . 0 H T M e P y P 2 5 5 3 n m 5 9 0 n m 0 . 9 0 . 8 5 4 2 n m 6 4 6 n m 4 1 7 n m 0 . 7 E u T M e P y P ( a c a c ) 0 . 6 5 5 0 n m 4 2 6 n m 0 . 5 E u T M e P y P ( a c a c ) 0 . 4 ( p a r t l y d e c o m p o s e d ) 0 . 3 0 . 2 0 . 1 3 0 0 3 2 5 3 5 0 3 7 5 4 0 0 4 2 5 4 5 0 4 7 5 5 0 0 5 2 5 5 5 0 5 7 5 6 0 0 6 2 5 6 5 0 6 7 5 7 0 0 [ n m ] Fig. 3. Absorption spectra of the: H2TMePyP (solid), Eu(III)TMePyP(acac) (dotted), and partially decomposed Eu(III)TMePyP(acac) (dashed lined) in monlithic sol-gel material The uv absorbance spectra illustrate the characteristic spectral changes that accompany porphyrin metallation. When we compare spectra of free base porphyrin with spectra of their Eu(III) complexes we can observed only 10 nm shift of Soret band, while dramatical changes in the Q band could be noticed. The Q band of the free base porphyrin consists of four components: Qx(0,0), Qx(1,0), Qy(0,0) and Qy(1,0) which are associated with D2h (mmm) symmetry while in the spectra of Eu(III) porphyrins [symmetry D4h (4 mmmm)] only one component Qy(0,0) is observed. The luminescence properties of porphyrin complexes with rare earth metals have been reported, but strong emission was observed only for Sc, Y, Gd, Lu and Yb. Here we report for the first time strong emission of Eu(III) porphyrin in monolithic silica. The fluorescence spectra of H2TMePyP and Eu complex are shown in Fig. 4. They are compared with the spectra of silica doted with europium chloride and with the complex which was partially decomposed. The compounds were excited with different wavelength. It can be noticed that strong fluorescence of europium porphyrin is observed under excitation in Soret band, while at the same time emission neither free-base porphyrin nor europium chloride does not occur. It can be explained by the strong interaction of the Eu(III)TMePyP(acac) wit the silica. 4 [a.u] 4 . 0 0 3 . 7 5 3 . 5 0 4 2 3 n m e x c .= 6 0 4 n m 6 5 3 n m 7 1 6 n m 3 . 2 5 6 5 4 n m 3 . 0 0 2 . 7 5 E u T M e P y P ( a c a c ) 2 . 5 0 p a r t l y d e c o m p . 2 . 2 5 Relativeintensityx10 2 . 0 0 1 . 7 5 E u C l 3 H T M e P y P 2 1 . 5 0 1 . 2 5 E u T M e P y P ( a c a c ) 1 . 0 0 0 . 7 5 6 5 0 n m 0 . 5 0 0 . 2 5 0 . 0 0 4 7 5 5 0 0 5 2 5 5 5 0 5 7 5 6 0 0 6 2 5 6 5 0 6 7 5 7 0 0 7 2 5 7 5 0 7 7 5 8 0 0 [ n m ] 4 [a.u] 4 . 0 4 4 3 n mE e x c .= u T M e P y P ( a c a c ) 3 . 5 3 . 0 5 5 6 n m 6 5 8 n m 2 . 5 H T M e P y P 2 Relativeintensityx10 2 . 0 1 . 5 E u T M e P y P ( a c a c ) 7 1 9 n m 6 2 0 n m p a r t l y d e c o m p . 6 5 2 n m 1 . 0 0 . 5 E u C l 3 0 . 0 4 7 5 5 0 0 5 2 5 5 5 0 5 7 5 6 0 0 6 2 5 6 5 0 6 7 5 7 0 0 7 2 5 7 5 0 7 7 5 8 0 0 [ n m ] 6 [a.u] 1 . 0 5 5 4 n m 0 . 9 0 . 8 u T M e P y P ( a c a c ) 5 3 0 n mE e x c .= p a r t l y d e c o m p . E u T M e P y P ( a c a c ) 0 . 7 E u C l 3 6 5 5 n m e m i s s i o n n o o b s e r v e d 0 . 6 Relativeintensityx10 0 . 5 7 1 5 n m 0 . 4 0 . 3 0 . 2 H T M e P y P 2 0 . 1 0 . 0 4 7 5 5 0 0 5 2 5 5 5 0 5 7 5 6 0 0 6 2 5 6 5 0 6 7 5 7 0 0 7 2 5 7 5 0 7 7 5 8 0 0 [ n m ] Fig. 4. Fluorescence spectra of the: H2TMePyP (solid), Eu(III)TMePyP(acac) (dotted), partially decomposed Eu(III)TMePyP(acac) (dashed lined) and EuCl3 (dash-dot line) in monolithic sol-gel material, excited at the various wavelengths. References 1. Milgrom L. R., “The colors of life: an introduction to the chemistry of porphyrins and related compounds”, Oxford University Press, UK, 1997. 2. R. Zusman, C. Rotman, and D. Avenir, J. Non-Cryst. Solids, 122 (1990) 107. 3. V. Bekiari, P. Lianos and P. Judenstein, Chem. Phys. Lett., 307 (1999) 310. 4. J.M Krüger and H.D. Breuer, Ber. Bunsenges. Phys. Chem., 11 (1998) 1554. 5. S.-K. Lee and I. Okura, Anal. Chim. Acta, 342 (1997) 181. 6. S.M. Arabei, Veret-Lamarnier and J.P. Galaup, Chem. Pys., 216 (1997) 163. 7. S. Radzki , P. Krausz, S. Gaspard, C. Giannotti, Inorg. Chimica Acta, 138, (1987) 139.