spectrophotometric investigation of the interaction between
advertisement

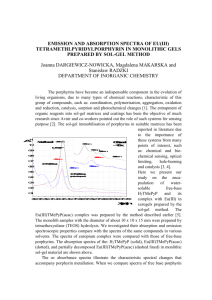
SPECTROPHOTOMETRIC INVESTIGATION OF THE INTERACTIONS BETWEEN PORPHYRINS AND CONCANAVALIN A Katarzyna POLSKA, Magdalena MAKARSKA and Stanisław RADZKI DEPARTMENT OF INORGANIC CHEMISTRY Porphyrins are a group of biologically important hydrophobic molecules. Naturally occurring porphyrins are bound to polypeptide chains, as can be seen in hemoglobin, mioglobina or chlorophyll. There have been numerous studies on the binding of synthetic porphyrins to proteins, lipids and other components of tissue. For example, porphyrins bind human serum albumin and low-density lipoproteins and can cause structural modifications [1-2]. Porphyrins could play a role of peptide receptors, which could bind peptides in protic solvents. Finding lectins with high affinity for porphyrins can open up new possibilities, such complexes can be employed in photodynamic therapy or as optical built-in sensors. Porphyrins preferentially can be accumulate in tumor tissue and upon irradiation by light of appropriate wavelength can react with molecular oxygen, sending it into its excited singled state, which in turn causes irreparable damage of cancerous tissue [3]. Some lectins exhibit a preferential agglutination of tumour cells; as a result, coupling porphyrins to lectins may increase selectivity of the conjugate for tumour cells. Concanavalin A, lectin of the jack bean (Canavalia ensiformis), was found in high concentration in growing tissues and may have role in the regulation of plant-cell division or germination via binding to non-polar growth factors [4]. Due to these properties concanavalin A can be considered as a potential carrier of porphyrin sensitizer to tumour tissues. The main purpose of our studies included examination of lectin-porphyrin complex formation using spectrophotometric titration as a leading analytical method. The present work was concerned on the two water-soluble cationic porphyrins: tetrakis[4-(trimethylammonio)phenyl] (H2TTMePP) and tetrakis (1-methyl-4pyridyl) (H2TMePyP). Absorption spectra were taken with a SPECORD M42 (Carl Zeiss Jena) spectrophotometer using quartz cells, to record spectra between 200 and 700 nm at the temp. of 21C. Spectra were registered digitally under the control of the program M500. We carried out UV-Vis titrations using H2TTMePP and H2TMePyP (concentration range about 10-6 M) with concanavalin A as a ligand (concentration range about 10-3 M) in the solution of TRIS buffer (0.025 M) in different values of pH. Addition of concanavalin A results in reduction of the Soret band intensity in the absorption spectra of porphyrin with a concomitant red shift of the band maximum. Non-linear least squares fitting of the observed absorbance changes in the Soret band region showed satisfactory fitting with 1:1 association model, however fitting with other stoichiometry was also performed. Absorbance in Soret Band 1.2 H2TMePyP + con A [TRIS] 1.0 0.8 pH = 0.96 logK = 3.76 pH = 2.74 logK = 3.87 pH = 8.50 logK = 5.78 pH = 10.45 logK = 5.70 0.6 0.4 0.2 0 50 100 150 200 250 300 CM conA * 106 Fig. 1. Plot of the absorbance change at the Soret band of H2TMePyP upon titration with concanavalin A in solutions of different pH; points are experimental; curves are generated from the Beck equation. For the calculation of the association constants (presented in the Fig 1) experimental data were fitted to the Beck equation, using non-linear regression method [5, 6]. The calculated constants are in good agreement with data known from the literature for the similar pairs of the interacting porphyrins and peptides [7]. As the conclusion of our preliminary studies we can say that, the cationic porphyrin can associate with concanavalin A, one molecule of lectin forms complex with one molecule of cationic porphyrin and the strength of association increases with increasing pH. The last observation can be explained by the various degree of porphyrin protonation in buffer with different pH. Our results also indicate that concanavalin A, relatively easy to extract from the plants, can potentially serve as supporting drug-delivery agent for porphyrin sensitizers in photodynamic therapy. References: [1] J. Davila, A. Harriman, J. Am. Chem. Soc. 112 (1990) 2686-2690. [2] J.P. Reyftmann, P.Morliere, S. Goldstein, R. Santus, L. Dubertret, D. Lagrange, Photochem. Photobiol. 40 (1984) 721-729. [3] B.A. Klyashchitsky, I.S. Nechaeva, G.V. Ponomaryov, J. Controlled Release 29 (1994) 1-16. [4] K.D. Hardman, C.F. Ainsworth, Biochemistry 12 (1973) 4442-4448. [5] M.T. Beck, Chemistry of Complex Equilibria; p. 93, Van Nostrand Reinhold Company, London (1970). [6] S. Radzki, P. Krausz, Monatshefte für Chemie 127 (1996) 51-61. [7] M. Sirish, H-J. Schneider, Chem. Commun. (1999) 907-908.