tem bulk
advertisement

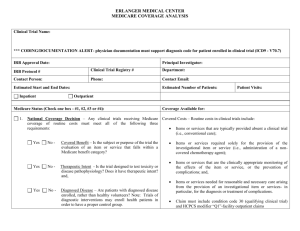
I. SETTING Health System II. DEFINITIONS For the purpose of this policy, "items and services" includes any drugs, biologics, devices or other items provided to the patient, any procedures done to the patient or specimens provided to the patient (for example, physician visits or drug injections.) III. GENERAL RULE FOR ALLOCATING RESEARCH RELATED COSTS A. Patient and insurers are not billed for items and services related to clinical trials, including: 1. The investigational item or service itself, including the provision and clinically appropriate monitoring of the investigational item or service, 2. Comparative drugs outside of normal use, 3. Items and services provided by the trial sponsors without charge, or paid for by the sponsors, 4. Items and services provided solely to satisfy data collection needs (protocol induced costs), and 5. Items and services needed for the diagnosis and treatment of complications related to the clinical trial. B. Items and services not billed to the patient are billed to the research bulk account. The principal investigator or the study's research coordinator is responsible for insuring that the patient is not charged for items that should be charged to the research bulk account. C. Exceptions to this general rule are listed in section V. IV. GENERAL PROCEDURES A. All principal investigators shall be educated about the policy. Any principal investigator who anticipates including UCDHS patients shall follow this procedure. B. Principal investigators will develop a separate list of all items and services required by or used by the study protocol. The list will include, when appropriate, the timing of such items and services relative to the entry of a subject in the protocol. Each item and service listed will be identified as follows: For Cancer Treatment Trials: 1. Bulk account (charged to bulk account)—any of the following: a. Investigational items or services. b. Protocol-induced items or services required solely to satisfy data collection needs c. Items and services paid for by the sponsors 2. Free (not charged to bulk account or payer)—Items provided by trial sponsor without charge. 3. Patient responsibility (charged to insurer)—any of the following, unless provided by the sponsor free of charge or paid for by the sponsor: a. Items or services required by protocol but expected to be needed in the course of normal patient care. b. Items or services required solely for the provision of the investigational item or service (i.e., administration of a non-covered chemotherapeutic agent), the clinically appropriate monitoring of the effects of the item or service or the prevention of complications. c. Items and services that are medically necessary for the diagnosis or treatment of complications arising from the provision of an investigational item or service. For trials that qualify for Medicare coverage of routine costs (see Section V for rules): 1. Bulk account (charged to bulk account)—any of the following: a. Investigational items or services. b. Protocol-induced items or services required solely to satisfy data collection needs c. Items and services paid for by the sponsors 2. Medicare/Bulk account (billable to Medicare, otherwise charged to bulk account)—any of the following, unless provided by the sponsor free of charge or paid for by the sponsor: a. Items or services required solely for the provision of the investigational item or service (i.e., administration of a noncovered chemotherapeutic agent), the clinically appropriate monitoring of the effects of the item or service or the prevention of complications. b. Items and services that are medically necessary for the diagnosis or treatment of complications arising from the provision of an investigational item or service. 3. Free (not charged to bulk account or payer)—Items provided by trial sponsor without charge. 4. Patient responsibility (charged to insurer)—Items or services required by protocol but expected to be needed in the course of normal patient care, unless provided by the sponsor free of charge or paid for by the sponsor. For all other trials: 1. Bulk account (charged to bulk account)—any of the following: a. Investigational items or services. b. Protocol-induced items or services required solely to satisfy data collection needs c. Items or services required solely for the provision of the investigational item or service (i.e., administration of a noncovered chemotherapeutic agent), the clinically appropriate monitoring of the effects of the item or service or the prevention of complications. d. Items and services that are medically necessary for the diagnosis or treatment of complications arising from the provision of an investigational item or service. d. Items and services paid for by the sponsors 2. Free (not charged to bulk account or payer)—Items provided by trial sponsor without charge. 3. Patient responsibility (charged to insurer)—Items or services required by protocol but expected to be needed in the course of normal patient care, unless provided by the sponsor free of charge or paid for by the sponsor. C. A copy of this list shall be placed in the patient's record immediately following the signed human subjects consent form. D. None of the listed items and services other than those labeled patient responsibility shall be charged to the patient. E. Any items and services labeled patient responsibility that in a particular patient's case end up not being needed in the course of that patient's care shall not be charged to that patient. F. Those items and services not charged to the patient shall be charged to the research bulk account. When such charges are incurred, the corresponding entry in the list shall be checked off. The clerk handling orders for the patient shall review the checklist so that charges destined for the bulk account will not be charged to the patient inadvertently. G. The principal investigator or the study's research coordinator shall review the research bulk account after research items or services are provided to ensure that no charges are missing. Any missing charges shall be reported to Patient Financial Services, so that the charge can be removed from the patient's account. V. SPECIAL RULES FOR MEDICARE AND FOR CANCER TREATMENT TRIALS A. Under California law, insurers must pay for the following clinical trial costs for cancer patients enrolled in a study that has a meaningful potential to benefit the patient. In addition, certain trials may qualify for Medicare coverage of the following clinical trials costs if they meet the standards in section V.B:. 1. Items or services that are typically provided absent a clinical trial. 2. Items or services required solely for the provision of the investigational item or service (i.e., administration of a non-covered chemotherapeutic agent), the clinically appropriate monitoring of the effects of the item or service or the prevention of complications. 3. Items and services that are medically necessary for the diagnosis or treatment of complications arising from the provision of an investigational item or service. B. Qualifying trial--In order to be covered by Medicare, the service must be part of a trial that meets all of the following criteria to be considered a qualifying trial: 1. Evaluates a Medicare benefit--the subject or purpose of the trial must be the evaluation of an item or service that falls within a Medicare benefit category (e.g., physicians' service, durable medical equipment, diagnostic test) and is not statutorily excluded from coverage (e.g., cosmetic surgery, hearing aids). 2. Has a therapeutic intent--the trial must have a therapeutic intent (i.e., is not designed exclusively to test toxicity or disease pathophysiology). 3. Enrolls diagnosed beneficiaries--trials of therapeutic interventions must enroll patients with diagnosed disease rather than healthy volunteers. Trials of diagnostic interventions may enroll healthy patients in order to have a proper control group. 4. Has Desirable Characteristics: a. Deemed trials--some trials are considered automatically deemed as having desirable characteristics. They include: (effective 9/19/200) 1) trials funded by the National Institute of Health (NIH, Centers for Disease Control and Prevention (CDC), Agency for Healthcare Research and Quality (AHRQ), HCFA, Department of Defense (DOD) and Department of Veterans Affairs (VA); 2) trials supported by centers or cooperative groups that are funded by the NIH, CDC, AHRQ, HCFA, DOD and VA; 3) trials conducted under an investigational drug application (IND) reviewed by the Food and Drug Administration (FDA); 4) drug trials that are exempt from having an IND under 21 CFR 312.2(b)(1) are deemed until the qualifying criteria are developed and the certification process is in place. At that time, the principal investigators of these trials must certify that the trials meet the qualifying criteria in order to maintain Medicare coverage of routine costs. This certification process will only affect the future status of the trial and will not be used to retroactively change the earlier deemed status. Until the Medicare clinical trials registry is established, the sponsors of both IND trials and IND-exempt trials must identify themselves by E-mail to clinicaltrials@hcfa.gov for administration, payment and program integrity purposes. b. Self-certified trials--in the future, a multi-agency Federal panel will develop qualifying criteria that will indicate a strong probability that a trial exhibits the desirable characteristics as stated in the NCD. No trials are covered based upon selfcertification at this time. DEVELOPED BY: Clinical Research Compliance Oversight Committee REFERENCES: Section 14132.98 of the California Welfare and Institutions Code HCFA program memorandum AB-00-89 REVIEWED BY: Ralph Green, MD Geneva Harris Bill McGowan Leslie Navarra Medical Staff Executive Committee Pharmacy and Therapeutics Committee