Transition Metals & Catalysis
advertisement
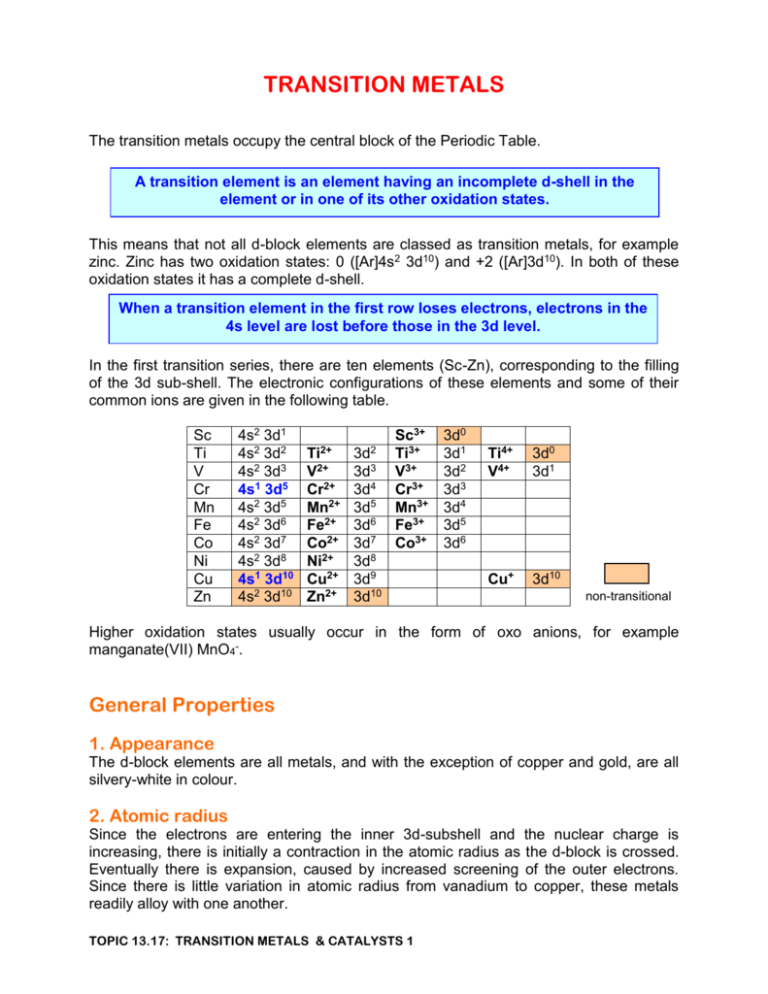
TRANSITION METALS The transition metals occupy the central block of the Periodic Table. A transition element is an element having an incomplete d-shell in the element or in one of its other oxidation states. This means that not all d-block elements are classed as transition metals, for example zinc. Zinc has two oxidation states: 0 ([Ar]4s2 3d10) and +2 ([Ar]3d10). In both of these oxidation states it has a complete d-shell. When a transition element in the first row loses electrons, electrons in the 4s level are lost before those in the 3d level. In the first transition series, there are ten elements (Sc-Zn), corresponding to the filling of the 3d sub-shell. The electronic configurations of these elements and some of their common ions are given in the following table. Sc Ti V Cr Mn Fe Co Ni Cu Zn 4s2 3d1 4s2 3d2 4s2 3d3 4s1 3d5 4s2 3d5 4s2 3d6 4s2 3d7 4s2 3d8 4s1 3d10 4s2 3d10 Ti2+ V2+ Cr2+ Mn2+ Fe2+ Co2+ Ni2+ Cu2+ Zn2+ 3d2 3d3 3d4 3d5 3d6 3d7 3d8 3d9 3d10 Sc3+ Ti3+ V3+ Cr3+ Mn3+ Fe3+ Co3+ 3d0 3d1 3d2 3d3 3d4 3d5 3d6 Ti4+ V4+ 3d0 3d1 Cu+ 3d10 non-transitional Higher oxidation states usually occur in the form of oxo anions, for example manganate(VII) MnO4-. General Properties 1. Appearance The d-block elements are all metals, and with the exception of copper and gold, are all silvery-white in colour. 2. Atomic radius Since the electrons are entering the inner 3d-subshell and the nuclear charge is increasing, there is initially a contraction in the atomic radius as the d-block is crossed. Eventually there is expansion, caused by increased screening of the outer electrons. Since there is little variation in atomic radius from vanadium to copper, these metals readily alloy with one another. TOPIC 13.17: TRANSITION METALS & CATALYSTS 1 3. Density The transition elements have small atomic radii and atomic volumes and high nuclear masses. As a result, their densities are high in comparison to the preceding s-block elements. 8 6 density g.cm-3 4 2 0 Sc Ti V Cr Mn Fe Co Ni Cu Zn 4. First Ionisation Energy The similarity of the d-block elements is exemplified by their similar ionisation energies. The slow increase in I1 across the d-block is caused by the increasing nuclear charge. 1st ionisation energy kJ.mol-1 800 400 0 Sc Ti V Cr Mn Fe Co Ni Cu Zn 5. Melting Point Owing to the large number of 3d and 4s electrons involved in the metallic bonding, these metals have high melting points. The exception is zinc, where only the 4s electrons are involved in bonding. All these metals have high electrical conductivities. TOPIC 13.17: TRANSITION METALS & CATALYSTS 2 3d Sc [Ar] Ti [Ar] V [Ar] Cr [Ar] Mn [Ar] Fe [Ar] Co [Ar] Ni [Ar] Cu [Ar] Zn [Ar] 4s As the outer 4s shell for all these atoms is occupied, they present a more or less similar face to the world. Similarities exist across the period as well as down the group. Cr and Cu have anomalous configurations, which arise because of the enhanced stability of half-filled dorbitals. This stability also explains why Fe2+ ([Ar]3d6) is easily oxidised to Fe3+ ([Ar]3d5) and Mn2+ ([Ar]3d5) is not easily oxidised to Mn3+ ([Ar]3d4) Characteristic properties of transition elements are: variable oxidation states formation of coloured ions formation of complexes catalytic activity TOPIC 13.17: TRANSITION METALS & CATALYSTS 3 1. Variable Oxidation States Except for scandium, which has exclusively an oxidation state of +3, the first transition series elements show an oxidation state of +2 (when both the 4s-electrons have been lost) and higher oxidation states, when 3d-electrons are involved. As the period is traversed, there is a trend towards decreased stability of the very high oxidation states and also the +2 oxidation state becomes more stable relative to the +3 oxidation state. In general, a +3 aqueous ion will oxidise the +2 aqueous ion of the element to the left of it. The exception is that Mn3+(aq) oxidises Fe2+(aq). The variable oxidation states displayed by transition metals are primarily due to the fact that successive ionisation energies of the atom increase gradually. For s and p block metals, there is a point at which there is a sharp increase in the values of successive ionisation energies. 12000 10000 8000 ionisation 6000 energy kJ.mol-1 I4 I3 I2 4000 I1 2000 0 Al V Cr Fe TOPIC 13.17: TRANSITION METALS & CATALYSTS 4 Zn The following oxidation states are known. The most stable oxidation state is circled; the most common oxidation states are in red. Sc 3 Ti V Cr Mn 7 Fe Co Ni 6 6 6 5 5 5 4 4 4 4 4 4 3 3 3 3 3 3 2 2 2 2 2 2 2 Cu Zn 2 2 1 In general, oxidation states lower than the most stable one are reducing agents, while those which are higher are oxidising agents. Oxidation states of vanadium Ammonium vanadate(V), NH4VO3, is a white solid. When added to dilute hydrochloric acid, the orange-yellow colour of the dioxovanadate(V) ion, VO2+, is formed. VO3- + 2H+ VO2+ + H2O There is no change in the oxidation state of vanadium. When this solution is shaken with zinc amalgam, colour changes take place over several minutes as vanadium is reduced through a number of oxidation states. VO2+(aq) + 2H+(aq) + eVO2+(aq) + 2H+(aq) + eV3+(aq) + e- VO2+(aq) + H2O blue V3+(aq) + H2O green V2+(aq) violet The final stage of the reduction, from vanadium(III) to vanadium(II), is best carried out under an atmosphere of nitrogen, since vanadium(II) ions are oxidized by oxygen in air. TOPIC 13.17: TRANSITION METALS & CATALYSTS 5 Oxidation states of chromium The highest oxidation state of chromium is +6, which is found in chromate(VI) and dichromate(VI) ions. The yellow chromate(VI) ion and the orange dichromate(VI) ion exist in a pH dependent equilibrium: in acid 2CrO42- + 2H+ Cr2O72- + H2O in alkali When acidified dichromate(VI) solution is shaken with zinc amalgam, colour changes take place as chromium is reduced through two of oxidation states. Cr2O72-(aq) + 14H+(aq) + 6eCr3+(aq) + e- 2Cr3+(aq) + 7H2O green Cr2+(aq) blue The final stage of the reduction, from chromium(III) to chromium(II), must be carried out under an atmosphere of nitrogen, since chromium(II) ions are readily oxidized by oxygen in air. Oxidation states of manganese The highest oxidation state of manganese is +7, which is found in manganate(VII) ions, MnO4-. This ion is an intense purple colour. When this solution is acidified and shaken with zinc amalgam, it is reduced directly to manganese(II) ions. [Mn(H2O)6]2+ ions are very pale pink and appear almost colourless in aqueous solution. MnO4-(aq) + 8H+(aq) + 5e- Mn2+(aq) + 4H2O(l) colourless Under alkaline conditions, manganate(VII) ions are reduced to manganese(IV) oxide which appears as a brown precipitate. MnO4-(aq) + 2H2O(l) + 3e- MnO2(s) + 4OH-(aq) Oxidation in alkaline solution Under alkaline conditions, transition metals in low oxidation states can be oxidised to higher oxidation states. For example: If sodium hydroxide solution is added to a solution of manganese(II) ions, the initial offwhite precipitate of manganese(II) hydroxide (Mn(OH)2) gradually darkens on standing as it is oxidised by air to manganese(IV) oxide (MnO2). TOPIC 13.17: TRANSITION METALS & CATALYSTS 6 Similarly, if sodium hydroxide solution is added to a solution of iron(II) ions, the initial dark green precipitate of iron(II) hydroxide (Fe(OH)2) goes brown fairly quickly on standing as it is oxidised by air to iron(III) hydroxide (Fe(OH)3). Oxidation is the loss of electrons. It is easier to lose an electron from a negative ion than from a neutral molecule; it is easier to lose an electron from a neutral molecule than from a positive ion. The metal-containing species present at different pH’s are: [M(H2O)6]2+ in acid solution [M(H2O)4(OH)2] neutral [M(OH4)]2in alkaline solution Clearly, oxidation will take place most easily under alkaline conditions. Alkaline oxidation: add an excess of sodium hydroxide solution add an oxidising agent, such as hydrogen peroxide An example is the oxidation of chromium(III). When excess NaOH is added to a solution of a chromium(III) salt, a dark green solution containing [Cr(OH)6]3- is formed. Treatment of this solution with hydrogen peroxide gives a yellow solution containing the chromate(VI) ion, CrO42-. 2[Cr(OH)6]3- + 3H2O2 2 CrO42- + 2OH- + 8H2O Another example is the reaction which occurs when concentrated ammonia solution is added to a pink solution of cobalt(II) ions until in excess. The initial green-blue precipitate of cobalt(II) hydroxide, Co(OH)2, re-dissolves to give a pale brown solution containing the hexaammine cobalt(II) ion. In the presence of air, the solution rapidly darkens and becomes dark brown as oxidation to the hexaammine cobalt(III) ion takes place. Although the hexaammine cobalt(III) ion is yellow, the mixture is dark because other species are present. [Co(H2O)6]2+ pink [Co(H2O)4(OH)2] green-blue [Co(NH3)6]2+ pale brown TOPIC 13.17: TRANSITION METALS & CATALYSTS 7 [Co(NH3)6]3+ yellow Catalysis A catalyst is a substance which alters the rate of a chemical reaction but is not used up in the reaction. Most catalysts are positive catalysts – they speed up chemical reactions. However, negative catalysts are sometimes used to retard undesirable reactions. For example, phosphoric acid is added to hydrogen peroxide to slow down the rate of its decomposition in light. A catalyst works by providing an alternative reaction path with a lower activation energy. Catalysts are usually divided into two classes: In heterogeneous catalysis, the catalyst and reactants are in different phases. In homogeneous catalysis, the catalyst and reactants are in the same phase. A catalyst increases the rate of attainment of equilibrium but does not alter the position of equilibrium. The efficiency of a catalyst is sometimes increased by adding a substance called a promoter, even though the promoter may have no catalytic activity in its own right. A substance which reduces the efficiency of a catalyst is called an inhibitor or a catalyst poison. Catalyst poisoning occurs with heterogeneous catalysts when, for example, sulphur compounds block the active sites; the sulphur is converted to inactive sulphides on the catalyst surface. Catalytic converters in cars are poisoned by any lead which is present in the petrol. Catalysts are important in improving the economics of industrial processes, since they allow products to be made in a shorter time. Transition metals are effective catalysts owing to their ability to exhibit different oxidation states and are widely used. Examples of transition elements as catalysts: Element Catalyst Reaction Ti V Cr Mn Fe Fe Co Ni Cu Pt TiCl4 / Al(C2H5)3 V2O5 Cr2O3 MnO2 Fe Fe2O3 Co Ni CuO Pt Ziegler-Natta polymerisation of alkenes Contact process CO + 2H2 CH3OH Decomposition of H2O2 Haber process Bosch process Oxo process: RCH=CH2 RCH2CH2CHO Catalytic hydrogenation of alkenes Deacon process: 4HCl + O2 2H2O + Cl2 Ostwald process; catalytic converters TOPIC 13.17: TRANSITION METALS & CATALYSTS 8 Heterogeneous Catalysis Heterogeneous catalysis typically involves gaseous reactants combining in the presence of a solid catalyst. Most industrial processes use heterogeneous catalysts. Heterogeneous catalysis can be regarded as involving three separate stages: 1. Adsorption In a simple gas phase reaction: X(g) + Y(g) Z(g) at least one of the reactants is adsorbed onto active sites on the surface of the catalyst by the formation of weak chemical bonds. This can result in an increase in reaction rate in a number of ways: X alone is adsorbed onto the surface of the catalyst, where it becomes effectively more concentrated. Molecules of X are therefore more likely to undergo collisions with Y than they would be purely in the gas phase - the frequency of collisions is increased existing chemical bonds may be weakened, making them easier to break - Ea is lowered X and Y are both adsorbed onto the surface of the catalyst, where they are in contact with each other at concentrations far greater than in the surrounding gas the frequency of collisions is increased the correct spacing of the active sites can ensure that molecules are held in the right geometry to react – Ea is lowered 2. Reaction The chemical reaction takes place on the surface of the catalyst. 3. Desorption The products of the reaction must be desorbed from the active sites to allow further molecules of reactant to be adsorbed. Preparing the catalyst Since a chemical reaction takes place only on the surface of the catalyst, increasing the surface area improves the efficiency of the catalyst. This is usually achieved by spreading the catalyst very thinly on an inert support medium. For example, in catalytic converters in cars, the very expensive mixed catalyst of platinum and rhodium is spread thinly onto a cheap ceramic support. The surface to mass ratio of the catalyst is greatly increased. Loss of catalyst from the surface of the support is a problem and can greatly increase the overall cost of the catalyst. Strength of Adsorption The strength of the adsorption onto the surface of the catalyst determines the effectiveness of the catalyst. If the adsorption is too weak, the rate of a reaction may not be greatly enhanced because: existing bonds are not weakened sufficiently molecules may not be held in the right geometry the concentration of reactant(s) on the catalyst surface is not increased enough TOPIC 13.17: TRANSITION METALS & CATALYSTS 9 Too weak adsorption tends to occur with metals near the end of a transition series, such as silver. Occasionally, weak adsorption can be advantageous, when one reaction is favoured above other competing and unwanted processes. An example of this is the silvercatalysed oxidation of ethene to epoxyethane. Reactant molecules must be free to move around on the surface of the catalyst in order to meet other reactant molecules and react. Products must be released fast enough to increase the overall reaction rate. If the adsorption is too strong, reactant molecules cannot move freely over the catalyst surface product molecules may not be desorbed from the surface of the catalyst quickly enough, if at all, blocking the active sites. The catalyst becomes poisoned by product molecules, and the reaction rate is not increased. Too strong adsorption tends to occur with metals at the beginning of a transition series, such as tungsten. Metals in the middle of a transition series, such as nickel and platinum, achieve a balance between the two effects and are good catalysts. Volcano curves show the change in catalytic activity across a transition series. x105 x x x104 x Relative reaction rate x103 x x102 x x x x x10 x1 x x Nb Cr Mo x Mn Tc Fe Ru TOPIC 13.17: TRANSITION METALS & CATALYSTS 10 Co Rh Ni Pd x Cu Specific Surface Catalysis Different distances between active sites in different catalysts and their different affinities for reactant molecules result in heterogeneous catalysts being specific. Different catalysts can bring about different reactions with the same reactant(s). For example: In the presence of a copper catalyst, ethanol is oxidised to ethanal. The adsorption of the O-H group weakens the hydrogen-oxygen bond, favouring dehydrogenation. CH3.CH2 O H Cu CH3 H H C H O Al2O3 In the presence of an aluminium oxide catalyst, ethanol reacts to form ethene. The adsorption of the C-O group weakens the carbon-oxygen bond, favouring dehydration. Examples of Heterogeneous Catalysis 1. Contact Process 2SO2(g) + O2(g) 2SO3(g) The catalyst for the reaction is vanadium(V) oxide, V2O5. During the reaction, vanadium changes its oxidation state; it is first reduced to vanadium(IV) by sulphur(IV) oxide and is then oxidised back to vanadium(V) by oxygen. SO2 + V2O5 SO3 + V2O4 2V2O4 + O2 2 V2O5 The oxidation state of vanadium is unchanged at the end of the reaction. This two-step process has a lower activation energy than the uncatalysed route. 2. Haber Process N2(g) + 3H2(g) 2NH3(g) The catalyst in the Haber process is iron. 3. Manufacture of methanol CO + 2H2 CH3OH The catalyst is Cr2O3 and the reaction takes place at 300atm and 300oC. TOPIC 13.17: TRANSITION METALS & CATALYSTS 11 Homogeneous Catalysis Homogeneous catalysis usually takes place in solution. When transition metal ions act as homogeneous catalysts, a change in oxidation state is usually involved, as the reaction proceeds via the formation of an intermediate species. An example of homogeneous catalysis is the oxidation of iodide ions by peroxodisulphate(VI) ions. The reaction is catalysed by either iron(II) or iron(III) ions. Despite a very favourable redox potential: 2I-(aq) + S2O82-(aq) I2(aq) + 2SO42-(aq) Eo = +1.51V the uncatalysed reaction is slow since it has to occur by the collision of two negativelycharged ions. In the presence of the catalyst, the activation energy is lower, because the reaction occurs by the collision of a negative ion with a positive ion. When the reaction is catalysed by an iron(II) catalyst, the iron(II) ions are first oxidised to iron(III) by peroxodisulphate(VI) ions. The iron(III) ions are then reduced back to iron(II) ions by iodide ions. 2Fe2+(aq) + S2O82-(aq) 2Fe3+(aq) + 2SO42-(aq) 2I-(aq) + 2Fe3+(aq) I2(aq) + 2Fe2+(aq) When the reaction is catalysed by an iron(III) catalyst, the two steps take place in reverse order. The iron(III) ions are first reduced to iron(II) ions by iodide ions; the iron(II) ions are then oxidised back to iron(III) by peroxodisulphate(VI) ions. 2I-(aq) + 2Fe3+(aq) I2(aq) + 2Fe2+(aq) 2Fe2+(aq) + S2O82-(aq) 2Fe3+(aq) + 2SO42-(aq) Autocatalysis In some reactions, one of the products of the reaction acts as a catalyst for the reaction. This is called autocatalysis. In such reactions, the reaction rate increases initially as the product is formed, instead of following the normal pattern of decreasing steadily. A plot of reaction rate against time shows a curve with a maximum. A plot of % reaction against time gives a characteristic sigmoid (s-shaped) curve. Reaction rate % reaction Time TOPIC 13.17: TRANSITION METALS & CATALYSTS 12 Time Examples: 1. Oxidation of ethanedioate ions by manganate(VII) ions. This reaction can be carried out as a titration at about 70 oC. It is catalysed by the Mn2+(aq) ions, which are formed in the reaction. The overall reaction is: 2MnO4- + 16H+ + 5C2O42- 2Mn2+ + 10CO2 + 8H2O When potassium manganate(VII) solution is first added from the burette to an acidified solution containing ethanedioate ions, the purple colour of manganate(VII) is not discharged immediately. However, once this colour has been discharged, forming Mn2+(aq) ions, the reaction is catalysed and further addition of manganate(VII) leads to an immediate discharge of the colour, which continues until the end-point is reached. The uncatalysed reaction is slow since it has to occur by the collision of two negativelycharged ions. In the presence of the catalyst, the activation energy is lower, because the reaction occurs, in two stages, by the collision of a negative ion with a positive ion. MnO4- + 8H+ +4Mn2+ 5Mn3+ + 4H2O 2Mn3+ + C2O42- 2Mn2+ + 2CO2 2. Oxidation of copper by concentrated nitric acid. The overall reaction is: Cu + 4HNO3 Cu(NO3)2 + 2NO2 + 2H2O The reaction is slow to begin with, until enough nitrogen dioxide has been formed to catalyse the reaction. TOPIC 13.17: TRANSITION METALS & CATALYSTS 13







