1 - The Royal Melbourne Hospital
advertisement

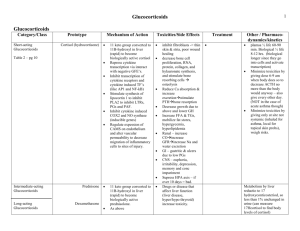
Melbourne Health Shared Pathology Service Cortisol (CORT) Specimens Test availability Reference intervals Adult, male & female Blood 5 ml, lithium-heparin or serum tube for cortisol blood levels. Urine, 24 h collection in a plain container for excretion studies. Saliva, collected in the pledget supplied for salivary levels. Assayed daily in blood; weekly in saliva and urine. Time, h Blood Saliva 0800-0900 1500-1600 150 - 650 120 - 400 2300 * * < 6.2 unit nmol/L nmol/L * no interval stated for this time Urinary excretion: 100 – 379 nmol/d Uncertainty of measurement: CVa: RMH, blood, 5.7% at 558 nmol/L; saliva, 27% at 14.9 nmol/L; urine, 6.2% at 179 nmol/L . Clinical applications and interpretation Blood assays Blood cortisol levels assess adrenocortical function in the response limb of a suppression or stimulation test. See the separate page for each dynamic test for advice on that procedure and the interpretation of results. Scroll down to find them. The random assay of cortisol in blood is only indicated as an initial test to exclude pituitary insufficiency or in the confirmation of Addisonian crisis. Salivary assays Salivary cortisol, which can be collected “at home” using the supplied collecting pledget, is particularly useful in establishing late evening trough levels; it similarly permits morning samples to be collected without having to attend a clinic. Midnight salivary cortisol collections are not used in night shift workers or patients with irregular sleeping hours. The MHSPS laboratory uses the Salivette pledget; no other device is accepted. Scroll down to find details of this test. The cortisol assayed in saliva is solely the free, (non protein-bound), hormone. Urine assays Excretion of cortisol in urine is used to demonstrate increased hormone secretion. The 24 h collection serves to integrate hormone clearance over the circadian cycle. Increases may occur with primary or with secondary adrenocortical hyperfunction, but may also be seen where the patient is obese, stressed or depressed, and with excess alcohol consumption. Urine cortisol levels are not used to evaluate adrenal hypofunction; the Synacthen stimulation test is appropriate there. Links References RCPA Manual of Test Use, Version 4. RCPA, Sydney, 2004. Reference intervals are derived from assay manufacturers, and have been confirmed by in-house studies. This page last reviewed and verified: 6 October 2010 by C Chiang. CHBIS-01,04 (06/10/10) Page 1 of 5 Melbourne Health Shared Pathology Service Cortisol (CORT) Clinical applications: How to use the Salivette. The MHSPS laboratory uses the Sarstedt Salivette pledget (O/N 51.1534) to permit patients to collect saliva for the assay of cortisol; no other device is accepted. For midnight salivary cortisol in the evaluation of Cushing’s Syndrome, one Salivette is used at 11 pm (2300 h). Salivettes are composed of an outer, conical shaped, tube with a plastic insert that contains a cotton swab or pledget. Collection procedure Plan ahead … Do not drink any citrus juices, e.g., orange juice, just prior to the collection. Fifteen (15) minutes before the collection rinse the mouth thoroughly with plain tap water. After the mouth rinsing, do not eat or drink anything else until the saliva collection is completed. The collection … Remove the plastic lid from the Salivette. Remove the cotton swab from the Salivette with your fingers. Place the swab in the mouth and leave it there for 3 minutes; you may move the swab around in your mouth but must not chew on it; thinking of lemon juice, just thinking, usually induces salivation. After 3 minutes spit the swab directly from your mouth into the Salivette, without handling it, and then put the supplied lid on. Label the Salivette with your name, hospital record number, date of birth, the date of the day when you did the collection, the time of collection. Store the capped Salivettes in the kitchen refrigerator at about 4o C. Finally, transport specimen to the laboratory the next morning. This page last reviewed and verified : 6 October 2010 by C Chiang. CHBIS-01,04 (06/10/10) Page 2 of 5 Melbourne Health Shared Pathology Service Cortisol (CORT) Dexamethasone suppression test, overnight (the 1mg dexamethasone suppression test) Clinical application This is the initial investigation when Cushing’s syndrome is suspected. Dexamethasone, a cortisol analogue, is administered to assess the negative feedback inhibition of ACTH secretion by the anterior pituitary gland. Dexamethasone is approximately 25 times as potent as cortisol. Usual total daily secretion of cortisol by the adrenal glands in the adult is approximately 7 to 10 mg. Requirements Dexamethasone, two 500 μg tablets, to make a 1 mg dose. No special patient preparation is needed; the test can be initiated “at home” provided blood samples can be drawn as required by the protocol. Protocol Day 1, 2200 – 2400 h: swallow dexamethasone, all the tablets at once. Day 2, 0800 – 0900 h: response cortisol, collect blood 5 ml, lithium-heparin or serum tube. Interpretation The day 2,morning cortisol should suppress,to less than 50 nmol/L. (Ref:J Clin Endocrinol Metab. 2008 May;93(5):1526-40). If day 2 cortisol is: < 50nmol/L, sensitivity 95%, specificity 80% < 140nmol/L, sensitivity 85%, specificity 95% Failure to suppress indicates that cortisol production is not responsive to normal feedback inhibitory mechanisms. Failure to suppress … occurs with Cushing’s syndrome, Cushing’s disease, exogenous steroid use and ectopic ACTH secretion … but may, however, also be seen in patients with endogenous depression, stress, or obesity, and with chronic excessive alcohol consumption … and as an effect of certain drugs which caused rapid metabolism of the oral dexamethasone dosee.g., phenytoin. Concealed failure to take the dexamethasone and abnormalities of dexamethasone absorption or metabolism should also be considered if there is a failure to suppress that is clinically incongruous. References RCPA Manual of Test Use, Version 4. RCPA, Sydney, 2004. Ismail AAA. Biochemical Investigations in Endocrinology. Methods and Interpretations. Academic Press, London, 1981. This page last reviewed and verified: 6 October 2010 by C Chiang. END OF PROTOCOL CHBIS-01,04 (06/10/10) Page 3 of 5 Melbourne Health Shared Pathology Service Cortisol (CORT) Dexamethasone suppression test (high dose suppression test) Clinical application This is the test used to determine the source of ACTH dependent Cushing’s syndrome. The test may be done according to the prolonged schedule described or as a high-dose, overnight test. Requirements Dexamethasone, 500 μg tablets. Protocol Dosing: the prolonged schedule Days 1 & 2, baseline assessment: Days 3 & 4, low dose medication: Days 5 & 6, high dose medication: Day 7, final assessment day: no steroid medication. dexamethasone, 500 μg, qid. dexamethasone, 2 mg, qid. no steroid medication. Sampling, every day for the 7 days Urine, 24 h collection into plain container for cortisol excretion. Blood, 0900 h, collect 5 ml, lithium-heparin or serum tube for cortisol. Blood, 0900 h, collect 10 ml, EDTA tube, immediately onto ice, for ACTH. Both blood samples must be sent to the laboratory immediately, each day. An alternative regime Alternatively, 8 mg of dexamethasone can be given as a single dose late in the evening. Interpretation of the cortisol response then moves immediately to the end of the schedule below and is done in conjunction with ACTH assay results and other investigations as needed. Interpretation Cortisol results Suppression is defined as a reduction of the morning cortisol level to <50% of the basal value or a post suppression cortisol level of < 140nmol/L. Suppression of cortisol levels on dexamethasone 2 mg/day decreases the likelihood of Cushing’s syndrome. Failure to suppress cortisol levels after 2 mg/day, but with suppression on 8 mg/day, indicates pituitary driven Cushing’s syndrome, conventionally termed Cushing’s disease. Failure to suppress cortisol on 8 mg/day indicates an adrenal neoplasm or the ectopic ACTH syndrome. Urine cortisol excretion results are used to support the diagnostic picture. ACTH results In Cushing’s disease, ACTH is suppressed by high dose dexamethasone. In the ectopic ACTH syndrome, ACTH is not suppressed. In adrenal neoplasia secreting cortisol, ACTH levels are low in the baseline specimen. Other modalities of investigation Pertinent scanning, and petrosal sinus blood sampling, are now also used to differentiate between a pituitary tumour and an ectopic source of ACTH. References RCPA Manual of Test Use, Version 4. RCPA, Sydney, 2004. Ismail AAA. Biochemical Investigations in Endocrinology. Methods and Interpretations. Academic Press, London, 1981. This page last reviewed and verified: 6 October 2010 by C Chiang. END OF PROTOCOL CHBIS-01,04 (06/10/10) Page 4 of 5 Melbourne Health Shared Pathology Service Cortisol (CORT) Synacthen stimulation tests Clinical application Investigation of suspected primary or secondary adrenocortical insufficiency. Assessment of possible adrenal suppression or atrophy due to steroid therapy. Synacthen® (generic: tetracosactrin) is a completely synthetic preparation of the first 24 of the 39 amino acids of the ACTH molecule. It is equipotent with endogenous ACTH. Requirements Synacthen, 250 μg in 1 mL ampoule; (Synacthen Depot, I mg x 3 if the long test is done). No special patient preparation is needed; the test should be performed before 10am so that the basal results can be interpreted. he facility to recover the patient from an anaphylaxis must be at hand during the test. Protocol Before the test: baseline cortisol, collect blood 5 ml, lithium-heparin or serum tube, baseline ACTH, EDTA tube on ice.. Inject Synacthen, 250 μg; injection may be either i.m. or i.v, At 30 and again at 60 minutes after injection: response cortisols, collect blood 5 ml, lithium-heparin or serum tube on both occasions. Interpretation In general A normal response in the short Synacthen test is demonstrated if post-stimulation sample’s cortisol itself exceeds 550 nmol/L. Failure to respond adequately suggests adrenal insufficiency. If baseline ACTH is also elevated, this then suggests primary adrenal failure. In the context of severe sepsis or intensive care patients: References RCPA Manual of Test Use, Version 4. RCPA, Sydney, 2004. Ismail AAA. Biochemical Investigations in Endocrinology. Methods and Interpretations. Academic Press, London, 1981. Annane D, Sébille V, Troché G, et al. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 2000; 238: 1038-45. This page last reviewed and verified : 6 October 2010 by C Chiang. END OF PROTOCOL CHBIS-01,04 (06/10/10) Page 5 of 5