Supplementary Materials and Methods (doc 554K)
advertisement

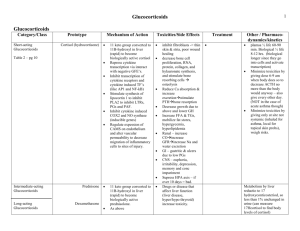
SUPPLEMENTARY MATERIALS AND METHODS Exclusion criteria During screening, participants were checked for psychiatric, neurological, cardiovascular or endocrine disease, irregular sleep/wake rhythm, non-admissibility to the MRI scanner, smoking (>5 cigarettes weekly), alcohol consumption (>21 beverages weekly), use of recreational drugs (>weekly), psychotropic medication, and hepatic, cardiovascular, or renal impairments. Athletes were excluded because of a possible positive doping test result after spironolactone intake. All participants had normal or correctedto-normal vision and normal hearing. They had to refrain from any medication other than paracetamol for acute pain and recreational drugs for 72h, alcohol for 24h, and coffee for 3h before testing. Stress measurements For negative mood, the Positive and Negative Affect Scale (PANAS, Watson et al, 1988) was administered either on paper or presented on the screen in the MRI scanner, programmed in Presentation® (Neurobehavioral Systems, Inc.). Sum scores for negative mood were calculated per time point. Vital signs were measured using an automatic blood pressure monitor with arm cuff (Intellisense®, OMRON, the Netherlands) outside the MRI scanner. Heart rate during scanning was acquired using the heart rate device of the MRI scanner, peak-scored using in-house software and averaged over four time bins of 1min to cover the task. To measure cortisol levels, saliva samples were taken using Salivettes® (Sarstedt, Germany). For each sample, participants were asked to chew the cotton swab gently for 1min. The samples were stored at -24°C until they were analyzed by the Dresden LabService (Germany). After thawing, the samples were centrifuged and analyzed using a commercially available chemiluminescence immunoassay with high sensitivity (IBL Inc.). The cortisol levels were not normally distributed and normalized using log-transformation. fMRI preprocessing For spatial realignment, head motion was estimated on the first echo using least-squares estimation and applied to the 5 echoes acquired for each excitation using 6 rigid-body transformation parameters. The echo images per volume were then combined into a single volume using an optimized echo weighting procedure (Poser et al, 2006). The functional images were coregistered to the structural image using rigid-body transformations. The T1-image was segmented into cerebral spinal fluid (CSF), white matter (WM) and gray matter and used to normalize functional and structural scans to MNI space with the unified segmentation procedure. Finally, the images were spatially smoothed using an 8mm FWHM Gaussian kernel. 1 SUPPLEMENTARY RESULTS Stress measures in the experiment phase Negative Mood. We found main effects of stress (F(1,91)=10.907, p=0.001) and time (F(2.4,218.4)=12.954, p<0.001), and a time-by-stress interaction (F(2.4,218.4)=9.812, p<0.001, Figure 1). Post-hoc tests revealed stronger negative mood in the stress groups at 5min (trend level, p=0.096) and 45min after stress induction (p<0.001) compared to the control groups. No other significant group differences were found. However, we found a trend for a time-by-stress-by-MR-blockade interaction (F(2.4,218.4)=2.692, p=0.060, Figure 1). Post-hoc tests revealed a time-by-stress interaction only in the MR-available groups (F(2.2,93.5)=10.269, p<0.001), but no significant stress-related increase in negative mood over time in the MR-blocked groups. It has been reported before, mostly in rodents, that the MR is involved in reactivity to novel situations, behavioral flexibility, and coping strategies (Berger et al, 2006; de Kloet et al, 1999; Oitzl & Dekloet, 1992; Oitzl et al, 1994). However, in humans this effect of MR-blockade on appraisal is not supported yet. Thus, we interpret our finding as first evidence supporting a role of the MR in appraisal of novel or stressful situations in humans, but a replication of this finding is needed. Cortisol. One participant from the Control/MR-blocked group was removed from this analysis because of excessive cortisol levels (both cortisol at 100min after stress and the increase of cortisol from 70 to 100min exceeded the mean + 3SD of the group). We found main effects of stress (F(1,92)=13.004, p=0.001) and MR-blockade (F(1,92)=15.013, p<0.001), time-by-stress (F(2.5,229.5)=8.927, p<0.001), and time-by-MRblockade (F(2.5,229.5)=6.217, p=0.001, Figure 1) interactions, but no time-by-stress-by-MR-blockade interaction. Post-hoc tests showed time-by-stress interactions in both drug groups (MR-available: F(2.5,114.4)=3.018, p=0.041; MR-blocked: F(2.3,105.6)=6.933, p=0.001). In the MR-available groups, the stress group had higher cortisol levels than the control group at 45min (p=0.044) and at trend level 70min after stress (p=0.085). Within the MR-blocked groups, stressed individuals had higher cortisol levels from 15min after stress onwards (all p<0.05). Drug effects were found from 5 to 100min, with the MR-available groups having lower cortisol levels than the MR-blocked groups (all p<0.05) in line with an impaired negative feedback in the MR-blocked group. No other significant group differences were found. Heart rate and blood pressure. We found main effects of stress (F(1,88)=4.665, p=0.033) and time (F(2.9,252.3)=14.721, p<0.001) on heart rate. Stressed participants had higher heart rates than control participants, indicative of heightened NE levels. No significant main effect of or interaction with MRblockade was present. Both systolic and diastolic blood pressure were unaffected by stress and MRavailability. Importantly, results did not change when raw data (log-transformed in the case of cortisol) and not difference scores were used. 2 SUPPLEMENTARY FIGURES Figure S1: Brain areas showing connectivity to the basolateral amygdala (BLA, left) or the centro-medial (CMA, right) during the emotional face-matching task, plottet on a template brain. All p<0.05, FWE corrected (peak level). 3 SUPPLEMENTARY REFERENCES Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM et al. (2006). Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proceedings of the National Academy of Sciences of the United States of America, 103, 195-200. de Kloet ER, Oitzl MS, & Joëls M. (1999). Stress and cognition: are corticosteroids good or bad guys? Trends in Neurosciences, 22, 422-426. Oitzl MS, & Dekloet ER. (1992). Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behavioral Neuroscience, 106, 62-71. Oitzl MS, Fluttert M, & de Kloet ER. (1994). The effect of corticosterone on reactivity to spatial novelty is mediated by central mineralocorticosteroid receptors. The European journal of neuroscience, 6, 1072-1079. Poser BA, Versluis MJ, Hoogduin JM, & Norris DG. (2006). BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: Parallel-acquired inhomogeneity-desensitized fMRI. Magnetic Resonance in Medicine, 55, 1227-1235. Watson D, Clark LA, & Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063-1070. 4