Bacterial Transformation: Creating E
advertisement

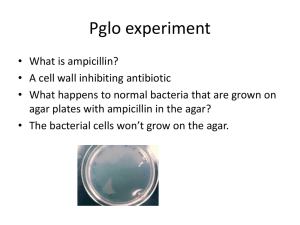
Name: Per: Bacterial Transformation: Creating E. coli that Glow PA Standards: 3.3.10C Describe how genetic information is inherited and expressed 3.3.12C Explain gene inheritance and expression at the molecular level Introduction Advances in biotechnology have given us the power to create organisms that would normally not occur in nature by manipulating DNA. In this lab, you will use a gene found in a jellyfish (Aequoria Victoria) that gives these jellyfish the ability to fluoresce and glow in the dark. You will take this gene and put it into cells of the common bacteria, E. coli. If the E. coli take up the gene and express it by making the protein, they will also glow in the dark under ultraviolet light! This process is called genetic transformation: the insertion of a new gene into an organism, in order to change the characteristics of the organism. Remember from your studies of DNA that: New DNA → New RNA → New Protein Produced → New Trait Why use E. coli? To genetically transform an entire organism, you must insert the new gene into every cell in the organism. Which organism would be most convenient for us to use? o One composed of many cells or one composed of a single cell? _____________ Once an organism has been genetically transformed, it should be able to pass on the new DNA (and the new trait) to all of its offspring. To determine if this is true, which type of organism would be most convenient for us to use? o Each new generation develops and reproduces quickly (hours or days) or each new generation develops and reproduces slowly (months or years)? _________________ Safety is another important consideration in choosing an experimental organism. If we are not sure what the effects of our experimental treatment will be, what characteristics would be important for the experimental organism to have (or not have)? _______________________________________________________________________ Based on the above considerations, which would the best choice for a genetic transformation? ○ Bacterium ○ Fruit fly ○ Mouse ○ Fish Explain your choice: ______________________________________________________________________ Bacteria are small and easily contained in petri dishes on growth medium called agar. They reproduce quickly; their population size doubles every 20 minutes under ideal conditions. They are also relatively inexpensive to obtain and keep alive. These cells can be killed with chemicals such as alcohol and bleach, as well as antibiotics such as penicillin and ampicillin. All of these characteristics make them ideal for use in a genetic transformation experiment. Petri dish with agar *Adapted from the pGLO™ Bacterial Transformation Kit, 166-0003EDU, Biotechnology Explorer (TM) instruction manual, Rev. E. copyright 2005 by Bio-Rad Laboratories, Life Science Education. 1-800-4BIORAD (800-424-6723), www.explorer.bio-rad.com pGLO Bacterial Transformation Rev. 5/22/07 Page 1 Science In Motion Susquehanna University Bacterial DNA Bacteria such as E. coli have no nucleus, but they do have a large, circular strand of DNA. In addition, they have tiny circles of “extra” DNA called plasmids. Plasmids usually contain a few genes that are beneficial for the bacterium, such as genes that allow the cell to resist and survive treatment with antibiotics. Bacteria are able to exchange plasmids, so they can pass these genes to other bacteria – even to bacteria of different species. This is one of the reasons bacteria can quickly develop resistance to antibiotics. DNA PLASMIDS BACTERIAL CELL WALL & MEMBRANE FLAGELLUM E. coli bacterium Plasmids make useful tools for genetic engineering Scientists discovered that they could use these plasmids as a vehicle to carry genes into bacterial cells, if they engineered the plasmids to contain the desired genes. They cut open the plasmids using restriction enzymes, add the chosen genes, then splice the plasmids closed. Under certain conditions, we can induce bacteria to take up the plasmids through their cell walls, and adopt the plasmids (along with the genes they carry) as part of their own genetic material. This is the basic procedure for genetic transformation, also known as genetic engineering. In this lab, you will use a plasmid called pGLO, which has been engineered to contain several genes that will be useful to us. Below is a “map” of the plasmid, showing the three genes that interest us. Ampr Gene for antibiotic resistance pGLO plasmid ARA Gene for turning on GFP when arabinose sugar is present GFP Gene for Green Fluorescent Protein; glows green under UV light pGLO Bacterial Transformation Rev. 5/22/07 Page 2 Science In Motion Susquehanna University Ampr Gene Genetic selection refers to the process whereby colonies can be selected for their ability to grow in various conditions. The Ampr gene codes for a protein that enables bacteria to survive treatment with the antibiotic ampicillin. Any bacterium that has this gene will be able to grow in the presence of ampicillin (ampicillin resistance), while bacteria without this gene will not. ARA and GFP Genes These genes work together to create the “glow-in-the-dark” effect. Gene expression in all organisms is carefully regulated to allow for adaptation to differing conditions and to prevent wasteful overproduction of unneeded proteins. When arabinose sugar is present in the bacterium’s environment, the ARA gene will be turned on. In normal bacteria, this gene initiates production of a protein that enables them to digest arabinose as a food source. For our plasmid, scientists have inserted this gene as an “on” switch for the GFP gene; when arabinose sugar is present, instead of initiating production of an arabinose enzyme, ARA activates the GFP gene so that the glow-in-the-dark protein is manufactured by the bacteria. If there is no arabinose sugar present in the bacteria’s environment, the GFP gene will not be transcribed and no glowing protein will be made. This mechanism is referred to as gene regulation. Adding genes to bacteria In order to get the engineered pGLO plasmid (and the gene to produce the Green Fluorescent Protein) into the E. coli bacteria, we have to perform several steps: Add pGLO plasmids to a CaCl2 solution containing E. coli cells. It is thought that the Ca2+ cation of the transformation solution neutralizes the repulsive negative charges of the phosphate backbone of the DNA and the phospholipids of the cell membrane, allowing the DNA to enter the cells. Heat shock the cells which increases the permeability of the cell membrane so the plasmids will pass through. Revive the cells that survive the heat shock process by incubating with nutrient broth to allow the bacteria to express the new genes. Plate the cells onto nutrient agar so the cells that received the plasmid can reproduce (passing on the new DNA to their offspring) and be seen. When we perform these steps, we will end up with millions of bacteria that survive the process, and only a few bacteria that have successfully received the pGLO plasmid. How might we separate the genetically modified bacteria from the ones that didn’t take up the new DNA? (hint: read over the features of the pGLO plasmid) ______________________________________________________________________________ ______________________________________________________________________________ Guiding Questions How can we use molecular biology to change the characteristics displayed by an organism? ______________________________________________________________________________ ______________________________________________________________________________ What ways do you think this technology could be applied in our daily life? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ pGLO Bacterial Transformation Rev. 5/22/07 Page 3 Science In Motion Susquehanna University Vocabulary Genetic transformation: the insertion of a new gene into an organism, in order to change the characteristics of the organism Plasmid: circles of “extra” self-replicating DNA usually containing a few genes that are beneficial for the bacterium; often convey antibiotic resistance Genetic selection: the process whereby colonies can be selected for their ability to grow in various conditions; antibiotic resistance is a type of genetic selection Agar: medium containing a nutrient mixture of carbohydrates, amino acids, nucleotides, salts, and vitamins that provides a solid matrix to support bacterial growth Green fluorescent protein (GFP): protein originally isolated from a bioluminescent jellyfish; the unique three-dimensional conformation of GFP causes it to resonate when exposed to ultraviolet light and give off energy in the form of visible green light Lawn: the appearance of bacterial colonies when all the individual colonies on a petri-dish agar plate merge together to form a field or mat of bacteria Gene regulation: the control of gene expression in all organisms to allow for differing conditions and to prevent wasteful overproduction of unneeded proteins; an example is the arabinose operon Materials Per lab group: 1 starter LB plate of E. coli strain: HB101 1 LB agar plate 2 LB/amp agar plates 1 LB/amp/ara agar plate 1 microtube sterile transformation buffer (CaCL2 solution) 1 microtube LB nutrient broth 2 sterile microtubes 7 sterile inoculation loops 1 100-1000 μl micropippettor 1 rack sterile pipette tips 1 foam microtube holder 1 container of crushed ice 1 waste container lined with biohazard bag 1 Sharpie marking pen 1 UV lamp 1 stopwatch 1 roll masking tape Per class: 1 vial rehydrated pGLO plasmid (on ice) 1 heat block set to 42° C 1 incubator set to 37° C Safety Students will employ proper sterile technique in the handling of bacteria. Proper hand washing at the beginning and conclusion of the laboratory is important. All materials coming in contact with bacteria should be placed in biohazard bags for decontamination. pGLO Bacterial Transformation Rev. 5/22/07 Page 4 Science In Motion Susquehanna University Procedure 1. Label one closed micro test tube +pGLO and another –pGLO. Place them in the foam rack. 2. Open the tubes and transfer 250 µl of transformation solution (CaC12) to each tube using the micropipettor and a sterile tip. 3. Use a sterile loop to pick up a single colony of bacteria from your starter plate. Pick up the +pGLO tube and immerse the loop into the transformation solution at the bottom of the tube. Spin the loop between your index finger and thumb until the entire colony is dispersed in the transformation solution (with no floating chunks of agar). Place the tube back in the tube rack. Using a new sterile loop, repeat for the –pGLO tube. 4. Place the tubes on ice. 5. Immerse a new sterile loop into the plasmid DNA stock tube. Withdraw a loopful. There should be a film of plasmid solution across the ring. This is similar to seeing a soapy film across a ring for blowing soap bubbles. Mix the loopful into the cell suspension of the +pGLO tube. Close the tube and return it to the foam rack on ice. Also close the –pGLO tube. Do not add plasmid DNA to the –pGLO tube. Why not? pGLO Bacterial Transformation Rev. 5/22/07 Page 5 Science In Motion Susquehanna University 6. Incubate the tubes on ice for 10 minutes. Make sure to push the tubes all the way down in the rack so the bottoms of the tubes stick out and make contact with the ice. 7. While the tubes are sitting on ice, examine the pGLO plasmid DNA solution with the UV lamp. Note your observations in Laboratory Observations: Day 1. Also label your four agar plates on the bottom (not the lid) as follows: Label one LB/amp plate: +pGLO; Label the LB/amp/ara plate: +pGLO; Label the other LB/amp plate: –pGLO; Label the LB plate: –pGLO. 8. Heat shock. Place both the +pGLO and –pGLO tubes directly into the heat block, set at 42 °C, for exactly 50 seconds. When the 50 seconds are done, place both tubes back in the foam rack on ice. For the best transformation results, the change from the ice (0°C) to 42°C and then back to be rapid. This step is critical for success! Then incubate tubes on ice for 2 minutes. Ice 42°C Heat Block 50 seconds Ice 9. Remove the rack containing the tubes from the ice and place on the bench top. Open a tube and, using a new sterile pipet tip, add 250 µl of LB nutrient broth to the tube and reclose it. Repeat with a new sterile pipet tip for the other tube. Incubate the tubes for 10 minutes at room temperature. 10. Tap the closed tubes with your finger to mix. Using a new sterile pipet tip for each tube, pipet 100 µl of the transformation, +pGLO, and control, –pGLO, suspensions onto the appropriate agar plates you labeled in step 7. 11. Use a new sterile loop for each plate. Spread the suspensions evenly around the surface of the agar by quickly skating the flat surface of a new sterile loop back and forth across the surface. 12. Stack up your plates and tape them together. Put your group name and class period on the tape and place the stack upside down in the 37°C incubator overnight. pGLO Bacterial Transformation Rev. 5/22/07 Page 6 Science In Motion Susquehanna University Bacterial Transformation: Creating E. coli that Glow Laboratory Observations: Day 1 1. Examine the E. coli bacteria growing on the stock culture plate. Describe: distribution of colonies on the plate: color of the colonies in room light: under UV light: size of the colonies: number of bacterial colonies (1 spot = 1 colony) on the plate: 2. Why do we heat shock the bacteria in the transformation solution? 3. Why do we add nutrient broth to the cells after heat shocking them? 4. Which plates serve as the control? What purpose does a control serve? 5. Predict what will happen on each of the plates: Will there be bacterial growth? Which plates will contain genetically transformed bacterial cells? Which plates will glow? -pGLO LB -pGLO LB+AMP pGLO Bacterial Transformation +pGLO LB+AMP Rev. 5/22/07 +pGLO LB+AMP+ARA Page 7 Science In Motion Susquehanna University Laboratory Observations: Day 2 Observe the results on your plates under normal room lighting; then hold the UV lamp over the plates and observe. Sketch what you observe on each plate and explain in the space beside the diagram why this happened. Describe the distribution of colonies on the plate, the color of the colonies in room light and under UV light, the size of the colonies, and the number of bacterial colonies (1 spot = 1 colony) on the plate as you did for the starter plate on day 1. -pGLO LB -pGLO LB+AMP +pGLO LB+AMP +pGLO LB+AMP+ARA The applications of this technology are far-reaching. Would you be in favor of using bacteria to produce proteins that could be used for medicine? How about genetically modified crops, to produce better-tasting or disease-resistant foods? What about genetically modified animals, to grow organs that wouldn’t be rejected by the human body, or pork with less fat? Would you be in favor of using technology that enabled us to cure diseases in babies before they are born? What about altering other characteristics, such as height, weight, or math ability? *Adapted from the pGLO™ Bacterial Transformation Kit, 166-0003EDU, Biotechnology Explorer (TM) instruction manual, Rev. E. copyright 2005 by Bio-Rad Laboratories, Life Science Education. 1-800-4BIORAD (800-424-6723), www.explorer.bio-rad.com pGLO Bacterial Transformation Rev. 5/22/07 Page 8 Science In Motion Susquehanna University Extension: Advanced Analysis of Results: Transformation Efficiency In many experiments, it is important to genetically transform as many cells as possible. For example, in some types of gene therapy, cells are collected from the patient, transformed in the laboratory, and then put back into the patient. The more cells that are transformed to produce the needed protein, the more likely the therapy will work. The transformation efficiency tells a researcher how effective the procedure is at getting DNA molecules into the bacterial cells. In this lab, the transformation efficiency represents the total number of bacterial cells that express the green protein, compared to the amount of DNA used in the experiment. You can calculate your group’s transformation efficiency in this lab by using the following formula: Transformation efficiency = Total number of cells growing on the LB/AMP/ARA plate Amount of plasmid DNA (in bacterial cells) spread on the agar plate Use the space below to calculate the transformation efficiency for your group. The units for transformation efficiency are transformants/g, and the numbers are usually large, so scientists frequently use scientific notation when describing these numbers. You will also need the following information about the plasmid DNA we used: 1. 10 l of pGLO DNA at a concentration of 0.08 g/l was added to the bacterial cells 2. 100 l of cells containing the pGLO were spread on the petri dish, from a total volume of 510 l of bacterial cells that received the pGLO DNA Number of colonies growing on LB/amp/ara plate: ______________ Amount of plasmid DNA in the bacterial cells spread on the plate: _____________ Using the above formula, our group’s transformation efficiency is: ______________ 1. Biotechnologists are in general agreement that the transformation protocol that you have just completed generally has a transformation efficiency of between 8.0 x 102 and 7.0 x 103 transformants per microgram of DNA. How does your transformation efficiency compare with these numbers? In the table below, report the transformation efficiency of the other teams in the class. Team Team Efficiency (tranformants/g) Efficiency (tranformants/g) 2. What might cause different groups to have different transformation efficiencies? pGLO Bacterial Transformation Rev. 5/22/07 Page 9 Science In Motion Susquehanna University Teacher Notes: pGLO Bacterial Transformation Time for completion: 2 x 45 minute periods separated by overnight incubation Target Grade Level: Advanced high school biology; AP Biology lab #6A Objectives Students will be able to describe, perform, and analyze the results of the genetic engineering technique of bacterial transformation and relate this knowledge to applications and impacts in today’s world. Major Concepts: Students should know what a gene is and should understand the relationship between genes and proteins. Students would also benefit from knowledge of the idea and practices of sterile technique, both for the success of the lab and for the safety of the students. The host organism for this lab is E. coli HB101, a nonpathogenic bacteria strain. Components of the lab that have been in contact with bacteria should be collected for sterilization. The lab requires lead time on the part of SIM to prepare the agar plates and an overnight incubation of starter plates before the day of the lab. The lab itself consists of a very full 42 minute period for day 1, an overnight incubation, and recording and analysis of results on day 2. Preparation: Nutrient agar plates have been prepared for this lab. These plates should be refrigerated in the plastic sleeves if several days precede the actual lab. Starter plates must be prepared 24-36 hours before the transformation laboratory. Using a sterile pipet, rehydrate the lyophilized E. coli HB101 by adding 250 μl of transformation solution directly to the vial. Recap the vial and allow the cell suspension to stand at room temperature for 5 minutes. Shake to mix before streaking on LB starter plates. Each lab team will need their own starter plate as a source of cells for the transformation. The plates should be streaked for single colonies as shown below: Place the plates upside down inside the incubator overnight at 37° C. Use for transformation within 24-26 hours. Do Not refrigerate before use. pGLO Bacterial Transformation Rev. 5/22/07 Page 10 Science In Motion Susquehanna University On the day of the lab prepare the pGLO plasmid by adding 250 μl of transformation solution into the vial of lyophilized pGLO plasmid DNA with a sterile pipet. If possible store the hydrated in the cold. Before the laboratory aliquot 1 ml of transformation solution (CaCl2) and 1 ml LB nutrient broth into separate color-coded 2 ml microtubes. Label the tubes. Each student work station should contain 1 starter plate, 4 poured agar plates (1 LB, 2 LB/amp, 1 LB/amp/ara), tube of transformation solution, tube of LB nutrient broth, inoculation loops, micropipettor, tips, foam rack, containers of ice, and a sharpie. The shared materials include the rehydrated plasmid DNA, the heat block set to 42° C, and the incubator set to 37° C. Typical results: –pGLO, LB plates: An even lawn of bacteria is present on this plate. The lawn appears offwhite. (this is the same plate condition and bacteria as the starter plate) –pGLO, LB/amp plates: No bacterial growth present on this plate. (no plasmid; no antibiotic resistence) +pGLO, LB/amp: Many transformed colonies of bacteria (optimally ~75). Colonies appear white. (Plasmid was taken up by these bacteria rendering them resistant to ampicillin and thus able to grow) +pGLO, LB/amp/ara: Many transformed colonies of bacteria (optimally ~75). Colonies appear white in the room light but appear bright fluorescent green when exposed to UV light. (Plasmid was taken up by these bacteria rendering them resistant to ampicillin and thus able to grow. Colonies glow because the presence of arabinose allows for the expression of the green fluorescent protein.) Sample calculation: Transformation efficiency 1. Determining the number of green fluorescent colonies: Count the colonies on the plate. (for numerator) 2. Determine the amount of pGLO plasmid DNA in the bacterial cells spread on the plates: a. Determine total amount of DNA DNA (μg) = (concentration of DNA ( μg/ μl) x (volume of DNA in μl) You used 10 μl of plasmid at a concentration of 0.08 μg/ μl. Total DNA = 0.08 μg/ μl x 10 μl = 0.8 μg total DNA b. Determine the fraction of this total DNA spread on each plate. Fraction of DNA used = volume spread on plate (100 μl) / total volume in tube (510 μl) 100 μl/510 μl = 0.2 c. Determine how many μg of DNA was spread on the plates: pGLO DNA spread (μg) = Total DNA used (0.8 μg) x fraction of DNA (0.2) = 0.16 μg plasmid DNA/plate (for denominator) 3. Determine the transformation efficiency Transformation efficiency = Total number of cells growing on the LB/AMP/ARA plate Amount of plasmid DNA (in bacterial cells) spread on the agar plate pGLO Bacterial Transformation Rev. 5/22/07 Page 11 Science In Motion Susquehanna University Answers to questions: 1. Examine the E. coli bacteria growing on the stock culture plate. Describe: distribution of colonies on the plate color of the colonies in room light and under UV light size of the colonies number of bacterial colonies (1 spot = 1 colony) on the plate. 2. Why do we heat shock the bacteria in the transformation solution? To increase the permeability of the cell membranes to permit the entry of the plasmid DNA 3. Why do we add nutrient broth to the cells after heat shocking them? To revive the cells that survive the heat shock process by incubating with nutrient broth to allow the bacteria to express the new genes 4. Which plates serve as the control? What purpose does a control serve? A control plate is a guide that is used to help you interpret the experimental results. Both –pGLO plates are control plates. The LB only –pGLO plate shows that bacteria were transferred and grew under the incubation conditions used. The LB/amp control –pGLO plate showing no growth in the presence of ampicillin is compared to the LB/amp +pGLO plate. This comparison shows that transformation produces colonies that can grow on ampicillin plates as the plasmid contains the ampicillin resistance gene. Without this control one would not know if the colonies on the LB/amp +pGLO plate were really transformants. Extension: The transformation efficiency section of the lab is optional and is appropriate for the AP lab. pGLO Bacterial Transformation Rev. 5/22/07 Page 12